Abstract
Review Article
The review of the relationship between UCP2 and obesity: Focusing on inflammatory-obesity
Mohamadreza Alivand*, Beitullah Alipour*, Sara Moradi, Yaser Khaje-Bishak and Maedeh Alipour
Published: 19 January, 2021 | Volume 5 - Issue 1 | Pages: 001-013
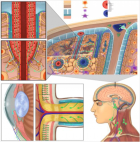
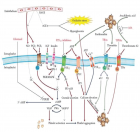
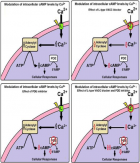
Understanding the obesity-related genes may provide future therapeutic strategies to modulate disease progression. UCP2 separates oxidative phosphorylation (OXPHOS) from ATP production in the inner mitochondria. Figure 1 shows the differences among UCP1, 2, 3. The main role of UCP2 is controlling the metabolism of energy in the cells [1-3]. Besides that, the expression of UCP2 is associated with chronic inflammation due to reactive oxygen species (ROS). In this regard, in injured cells and tissues, ROS could be decreased by reducing the proton motor force by the anti-inflammatory effect of UCP2 [4].
Read Full Article HTML DOI: 10.29328/journal.niogb.1001015 Cite this Article Read Full Article PDF
Keywords:
UCP2; Metabolism; Obesity; ROS; Inflammation
References
- Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med. 2013; 34: 465-484. PubMed: https://pubmed.ncbi.nlm.nih.gov/23266187/
- Bouillaud F. UCP2, not a physiologically relevant uncoupler but a glucose sparing switch impacting ROS production and glucose sensing. Biochim Biophys Acta. 2009; 1787: 377-83. PubMed: https://pubmed.ncbi.nlm.nih.gov/19413946/
- Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, et al. A signalling role for 4‐hydroxy‐2‐nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003; 22: 4103-4110. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC175801/
- Mehta SL, Li A. Neuroprotective role of mitochondrial uncoupling protein 2 in cerebral stroke. J Cereb Blood Flow Metab. 2009; 29: 1069-1078. PubMed: https://pubmed.ncbi.nlm.nih.gov/19240738/
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007; 132: 2169-2180. PubMed: https://pubmed.ncbi.nlm.nih.gov/17498510/
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009; 6: 399-409. PubMed: https://pubmed.ncbi.nlm.nih.gov/19399028/
- Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012; 56: 704-713. PubMed: https://pubmed.ncbi.nlm.nih.gov/22120206/
- Mathieu P, Lemieux I, Després JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010; 87: 407-416. PubMed: https://pubmed.ncbi.nlm.nih.gov/20200516/
- Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014; 15: 6184-6223. PubMed: https://pubmed.ncbi.nlm.nih.gov/24733068/
- Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015; 3: 207-215. PubMed: https://pubmed.ncbi.nlm.nih.gov/25066177/
- Ali AS, Ali S, Ahmad A, Bao B. Philip PA, et al. Expression of microRNAs: potential molecular link between obesity, diabetes and cancer. Obesity Reviews. 2011; 12: 1050-1062.
- Grossmann ME, Cleary MP. The balance between leptin and adiponectin in the control of carcinogenesis–focus on mammary tumorigenesis. Biochimie. 2012; 94: 2164-2171. PubMed: https://pubmed.ncbi.nlm.nih.gov/22728769/
- Divella R, De Luca R, Abbate I, Naglieri E, Daniele A, et al. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer. 2016; 7: 2346. PubMed: https://pubmed.ncbi.nlm.nih.gov/27994674/
- Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010; 15: 556-565. PubMed: https://pubmed.ncbi.nlm.nih.gov/20507889/
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004; 4: 579-591. PubMed: https://pubmed.ncbi.nlm.nih.gov/15286738/
- Van Kruijsdijk RC, Van Der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009; 18: 2569-2578. PubMed: https://pubmed.ncbi.nlm.nih.gov/19755644/
- Renehan AG, Roberts DL, Dive C, Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008; 114: 71-83. PubMed: https://pubmed.ncbi.nlm.nih.gov/18465361/
- Lee YH, Kim W, Yu BC, Park BL, Kim LH, et al. Association of the ins/del polymorphisms of uncoupling protein 2 (UCP2) with BMI in a Korean population. Biochem Biophys Res Commun. 2008; 371: 767-771. PubMed: https://pubmed.ncbi.nlm.nih.gov/18460338/
- Yoon Y, Park BL, Cha MH, Kim KS, Cheong HS, et al. Effects of genetic polymorphisms of UCP2 and UCP3 on very low calorie diet-induced body fat reduction in Korean female subjects. Biochem Biophys Res Commun. 2007; 359: 451-456. PubMed: https://pubmed.ncbi.nlm.nih.gov/17544366/
- Krempler F, Esterbauer H, Weitgasser R, Ebenbichler C, Patsch JR, et al. A functional polymorphism in the promoter of UCP2 enhances obesity risk but reduces type 2 diabetes risk in obese middle-aged humans. Diabetes. 2002; 51: 3331-3335. PubMed: https://pubmed.ncbi.nlm.nih.gov/12401727/
- Choi J, Kim KJ, Koh EJ, Lee BY. Gelidium elegans regulates the AMPK-PRDM16-UCP-1 pathway and has a synergistic effect with orlistat on obesity-associated features in mice fed a high-fat diet. Nutrients. 2017; 9: 342. PubMed: https://pubmed.ncbi.nlm.nih.gov/28358328/
- Mao L, et al. Long-chain polyunsaturated fatty acids and extensively hydrolyzed casein-induced browning in a Ucp-1 reporter mouse model of obesity. Food Funct. 2018; 9: 2362-2373. PubMed: https://pubmed.ncbi.nlm.nih.gov/29589625/
- Boss O, Hagen T, Lowell BB. Uncoupling proteins 2 and 3: potential regulators of mitochondrial energy metabolism. Diabetes. 2000; 49: 143-156. PubMed: https://pubmed.ncbi.nlm.nih.gov/10868929/
- Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, et al. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997; 15: 269-272. PubMed: https://pubmed.ncbi.nlm.nih.gov/9054939/
- Liu Y, Jiang H, He LY, Huang WJ, He XY, et al. Abnormal expression of uncoupling protein-2 correlates with CYP11A1 expression in polycystic ovary syndrome. Reprod Fertil Dev. 2011; 23: 520-526. PubMed: https://pubmed.ncbi.nlm.nih.gov/21557918/
- Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 2012; 10: e1001314. PubMed: https://pubmed.ncbi.nlm.nih.gov/22545021/
- Malli R, Graier WF. The Role of Mitochondria in the Activation/Maintenance of SOCE: The Contribution of Mitochondrial Ca(2+) Uptake, Mitochondrial Motility, and Location to Store-Operated Ca(2+) Entry. Adv Exp Med Biol. 2017; 993: 297-319. PubMed: https://pubmed.ncbi.nlm.nih.gov/28900921/
- Cannon B, Shabalina IG, Kramarova TV, Petrovic N, Nedergaard J. Uncoupling proteins: a role in protection against reactive oxygen species--or not? Biochim Biophys Acta. 2006; 1757: 449-458. PubMed: https://pubmed.ncbi.nlm.nih.gov/16806053/
- Nedergaard J, Cannon B. The 'novel' 'uncoupling' proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp Physiol. 2003; 88: 65-84. PubMed: https://pubmed.ncbi.nlm.nih.gov/12525856/
- Klaus S, Pültz S, Thöne-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes (Lond). 2005; 29: 615-623. PubMed: https://pubmed.ncbi.nlm.nih.gov/15738931/
- Nakayama K, Miyashita H, Yanagisawa Y, Iwamoto S. Seasonal effects of UCP1 gene polymorphism on visceral fat accumulation in Japanese adults. PLoS One. 2013; 8: e74720. PubMed: https://pubmed.ncbi.nlm.nih.gov/24086366/
- Porter RK. A new look at UCP 1. Biochim Biophys Acta. 2006; 1757: 446-448.
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, et al. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001; 1504: 82-106. PubMed: https://pubmed.ncbi.nlm.nih.gov/11239487/
- Esteves TC, Brand MD. The reactions catalysed by the mitochondrial uncoupling proteins UCP2 and UCP3. Biochim Biophys Acta. 2005; 1709: 35-44. PubMed: https://pubmed.ncbi.nlm.nih.gov/16005426/
- Rousset S, Mozo J, Dujardin G, Emre Y, Masscheleyn S, et al. UCP2 is a mitochondrial transporter with an unusual very short half‐life. FEBS Lett. 2007; 581: 479-482. PubMed: https://pubmed.ncbi.nlm.nih.gov/17240372/
- Zaninovich A. Role of uncoupling proteins UCP1, UCP2 and UCP3 in energy balance, type 2 diabetes and obesity. Synergism with the thyroid. Medicina. 2005; 65: 163-169. PubMed: https://pubmed.ncbi.nlm.nih.gov/16075814/
- Hassan NE, El-Masry SA, Zarouk W, El Banna EA, Mosaad RM, et al. Obesity phenotype in relation to gene polymorphism among samples of Egyptian children and their mothers. Genes Dis. 2018; 5: 150-157. PubMed: https://pubmed.ncbi.nlm.nih.gov/30258944/
- Vogler S, Goedde R, Miterski B, Gold R, Kroner A, et al. Association of a common polymorphism in the promoter of UCP2 with susceptibility to multiple sclerosis. J Molecular Med. 2005; 83: 806-811. PubMed: https://pubmed.ncbi.nlm.nih.gov/16021520/
- Rudofsky G, Schroedter A, Schlotterer A, Voron'ko OE, Schlimme M, et al. Functional polymorphisms of UCP2 and UCP3 are associated with a reduced prevalence of diabetic neuropathy in patients with type 1 diabetes. Diabetes Care. 2006; 29: 89-94. PubMed: https://pubmed.ncbi.nlm.nih.gov/16373902/
- Hurtaud C, Gelly C, Bouillaud F, Lévi-Meyrueis C. Translation control of UCP2 synthesis by the upstream open reading frame. Cell Mol Life Sci. 2006; 63: 1780-1789. PubMed: https://pubmed.ncbi.nlm.nih.gov/16845607/
- Beck V, Jabůrek M, Demina T, Rupprecht A, Porter RK, et al. Polyunsaturated fatty acids activate human uncoupling proteins 1 and 2 in planar lipid bilayers. FASEB J. 2007; 21: 1137-1144. PubMed: https://pubmed.ncbi.nlm.nih.gov/17242157/
- Reilly JM, Thompson MP. Dietary fatty acids up-regulate the expression of UCP2 in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 2000; 277: 541-545. PubMed: https://pubmed.ncbi.nlm.nih.gov/11061990/
- Rieusset J, Auwerx J, Vidal H. Regulation of gene expression by activation of the peroxisome proliferator-activated receptor γ with rosiglitazone (BRL 49653) in human adipocytes. Biochem Biophys Res Commun. 1999; 265: 265-271. PubMed: https://pubmed.ncbi.nlm.nih.gov/10548525/
- Bugge A, Siersbaek M, Madsen MS, Göndör A, Rougier C, et al. A novel intronic peroxisome proliferator-activated receptor γ enhancer in the Uncoupling Protein (UCP) 3 gene as a regulator of both UCP2 and-3 expression in adipocytes. J Bio Chem. 2010. 285: 17310-17317. PubMed: https://pubmed.ncbi.nlm.nih.gov/20360005/
- Oberkofler H, Klein K, Felder TK, Krempler F, Patsch W, et al. Role of peroxisome proliferator-activated receptor-γ coactivator-1α in the transcriptional regulation of the human uncoupling protein 2 gene in INS-1E cells. Endocrinology. 2006; 147: 966-976. PubMed: https://pubmed.ncbi.nlm.nih.gov/16282353/
- Lin J, Yang R, Tarr PT, Wu P, Handschin C, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell. 2005; 120: 261-273. PubMed: https://pubmed.ncbi.nlm.nih.gov/15680331/
- Medvedev AV, Robidoux J, Bai X, Cao W, Floering LM, et al. Regulation of the uncoupling protein-2 gene in INS-1 β-cells by oleic acid. J Biol Chem. 2002; 277: 42639-42644. PubMed: https://pubmed.ncbi.nlm.nih.gov/12205102/
- Azzu V, Brand MD. The on-off switches of the mitochondrial uncoupling proteins. Trends Biochem Sci. 2010. 35: 298-307. PubMed: https://pubmed.ncbi.nlm.nih.gov/20006514/
- Mailloux RJ, Seifert EL, Bouillaud F, Aguer C, Collins S, et al. Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem. 2011; 286: 21865-21875. PubMed: https://pubmed.ncbi.nlm.nih.gov/21515686/
- Chen H, Chan DC. Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab. 2017; 26: 39-48. PubMed: https://pubmed.ncbi.nlm.nih.gov/28648983/
- Richard D, Rivest R, Huang Q, Bouillaud F, Sanchis D, et al. Distribution of the uncoupling protein 2 mRNA in the mouse brain. J Comp Neurol. 1998; 397: 549-560. PubMed: https://pubmed.ncbi.nlm.nih.gov/9699915/
- Barnstable CJ, Reddy R, Li H, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) regulates retinal ganglion cell number and survival. J Molecular Neurosci. 2016; 58: 461-469. PubMed: https://pubmed.ncbi.nlm.nih.gov/26846222/
- Chen X, Wang K, Chen J, Guo J, Yin Y, et al. In vitro evidence suggests that miR-133a-mediated regulation of uncoupling protein 2 (UCP2) is an indispensable step in myogenic differentiation. J Biol Chem. 2009; 284: 5362-5369. PubMed: https://pubmed.ncbi.nlm.nih.gov/19073597/
- Sun LL, Jiang BG, Li WT, Zou JJ, Shi YQ, et al. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res Clin Pract. 2011; 91: 94-100. PubMed: https://pubmed.ncbi.nlm.nih.gov/21146880/
- Di Castro S, Scarpino S, Marchitti S, Bianchi F, Stanzione R, et al. Differential modulation of uncoupling protein 2 in kidneys of stroke-prone spontaneously hypertensive rats under high-salt/low-potassium diet. Hypertension. 2013; 61: 534-541. PubMed: https://pubmed.ncbi.nlm.nih.gov/23297375/
- Jin X, Chen D, Zheng RH, Zhang H, Chen YP, et al. miRNA-133a-UCP2 pathway regulates inflammatory bowel disease progress by influencing inflammation, oxidative stress and energy metabolism. World J Gastroenterol. 2017; 23: 76-86. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5221288/
- Jiang L, Qiu W, Zhou Y, Wen P, Fang L, et al. A microRNA-30e/mitochondrial uncoupling protein 2 axis mediates TGF-β1-induced tubular epithelial cell extracellular matrix production and kidney fibrosis. Kidney Int. 2013; 84: 285-296. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3731557/
- Muhammad HFL, Sulistyoningrum DC, Huriyati E, Lee YY, Wan Muda WAM. The Interaction between Coffee: Caffeine Consumption, UCP2 Gene Variation, and Adiposity in Adults-A Cross-Sectional Study. J Nutriti Metabol. 2019; 2019: 9606054. PubMed: https://pubmed.ncbi.nlm.nih.gov/30719347/
- Emre Y, Hurtaud C, Nübel T, Criscuolo F, Ricquier D, et al. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J. 2007; 402: 271-278. PubMed: https://pubmed.ncbi.nlm.nih.gov/17073824/
- Tagen M, Elorza A, Kempuraj D, Boucher W, Kepley CL, et al. Mitochondrial uncoupling protein 2 inhibits mast cell activation and reduces histamine content. J Immunol. 2009; 183: 6313-6319. PubMed: https://pubmed.ncbi.nlm.nih.gov/19846869/
- Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, et al. Persistent nuclear factor-κB activation in Ucp2-/-mice leads to enhanced nitric oxide and inflammatory cytokine production. J Bio Chem. 2005; 280: 19062-19069. PubMed: https://pubmed.ncbi.nlm.nih.gov/15757894/
- Su J, Liu J, Yan XY, Zhang Y, Zhang JJ, et al. Cytoprotective effect of the UCP2-SIRT3 signaling pathway by decreasing mitochondrial oxidative stress on cerebral ischemia–reperfusion injury. Int J Molecular Sci. 2017; 18: 1599. PubMed: https://pubmed.ncbi.nlm.nih.gov/28737710/
- Boschmann M, Thielecke F. The effects of epigallocatechin-3-gallate on thermogenesis and fat oxidation in obese men: a pilot study. J Am College Nutrit. 2007; 26: 389S-395S. PubMed: https://pubmed.ncbi.nlm.nih.gov/17906192/
- Schutz Y, Bessard T, Jequier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr. 1984; 40: 542-552. PubMed: https://pubmed.ncbi.nlm.nih.gov/6540980/
- Diepvens K, Westerterp KR, Westerterp-Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am J Physiol Regulat Integrat Comparat Phy. 2007; 292: R77-R85. PubMed: https://pubmed.ncbi.nlm.nih.gov/16840650/
- Tentolouris N, et al. Diet-induced thermogenesis and substrate oxidation are not different between lean and obese women after two different isocaloric meals, one rich in protein and one rich in fat. Metabolism. 2008; 57: 313-320. PubMed: https://pubmed.ncbi.nlm.nih.gov/18249201/
- Toda C, Diano S, Mitochondrial UCP2 in the central regulation of metabolism. Best Pract Res Clin Endocrinol Metab. 2014. 28: 757-764. PubMed: https://pubmed.ncbi.nlm.nih.gov/25256770/
- Dmitriev RI, Papkovsky DB. In vitro ischemia decreases histone H4K16 acetylation in neural cells. FEBS Letters. 2015; 589: 138-144. PubMed: https://pubmed.ncbi.nlm.nih.gov/25479088/
- Mattiasson G, et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003; 9: 1062-1068. PubMed: https://pubmed.ncbi.nlm.nih.gov/12858170/
- Myers Jr, MG, Olson DP. Central nervous system control of metabolism. Nature. 2012; 491: 357. PubMed: https://pubmed.ncbi.nlm.nih.gov/23151578/
- Horvath TL, Warden CH, Hajos M, Lombardi A, Goglia F, et al. Brain uncoupling protein 2: uncoupled neuronal mitochondria predict thermal synapses in homeostatic centers. J Neurosci. 1999; 19: 10417-10427. PubMed: https://pubmed.ncbi.nlm.nih.gov/10575039/
- Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, et al. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metabol. 2007; 5: 21-33. PubMed: https://pubmed.ncbi.nlm.nih.gov/17189204/
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, β cell dysfunction, and type 2 diabetes. Cell. 2001; 105: 745-755. PubMed: https://pubmed.ncbi.nlm.nih.gov/11440717/
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007; 449: 228-232. PubMed: https://pubmed.ncbi.nlm.nih.gov/17728716/
- Allister EM, Robson-Doucette CA, Prentice KJ, Hardy AB, Sultan S, et al. UCP2 regulates the glucagon response to fasting and starvation. Diabetes. 2013; 62: 1623-1633. PubMed: https://pubmed.ncbi.nlm.nih.gov/23434936/
- Diano S, Liu ZW, Jeong JK, Dietrich MO, Ruan HB, et al. Peroxisome proliferation–associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med. 2011; 17: 1121-1127. PubMed: https://pubmed.ncbi.nlm.nih.gov/21873987/
- Dulloo AG, Seydoux J, Jacquet J. Adaptive thermogenesis and uncoupling proteins: a reappraisal of their roles in fat metabolism and energy balance. Physiol Behav. 2004; 83: 587-602. PubMed: https://pubmed.ncbi.nlm.nih.gov/15621064/
- Rafiq R, El Haddaoui H, de Mutsert R, Rosendaal FR, Hiemstra PS, et al. Adiposity is a confounding factor which largely explains the association of serum vitamin D concentrations with C-reactive protein, leptin and adiponectin. Cytokine. 2020; 131: 155104. PubMed: https://pubmed.ncbi.nlm.nih.gov/32325367/
- Rezazadeh L, Gargari BP, Jafarabadi MA, Alipour B. Effects of probiotic yogurt on glycemic indexes and endothelial dysfunction markers in patients with metabolic syndrome. Nutrition. 2019; 62: 162-168. PubMed: https://pubmed.ncbi.nlm.nih.gov/30921552/
- Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González A, et al. Inflammation, oxidative stress, and obesity. Int J Molecular Sci. 2011; 12: 3117-3132. PubMed: https://pubmed.ncbi.nlm.nih.gov/21686173/
- Chan JL, Heist K, De Paoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Investigat. 2003; 111: 1409-1421. PubMed: https://pubmed.ncbi.nlm.nih.gov/12727933/
- Wasim M, Awan FR, Najam SS, Khan AR, Khan HN. Role of leptin deficiency, inefficiency, and leptin receptors in obesity. Biochem Genetics. 2016; 54: 565-572. PubMed: https://pubmed.ncbi.nlm.nih.gov/27313173/
- Scarpace P, Nicolson M, Matheny M. UCP2, UCP3 and leptin gene expression: modulation by food restriction and leptin. J Endocrinol. 1998; 159: 349-357. PubMed: https://pubmed.ncbi.nlm.nih.gov/9795377/
- Ho WL, Liu HF, Ho HW, Zhang WY, Chu AC, et al. Mitochondrial uncoupling protein-2 (UCP2) mediates leptin protection against MPP+ toxicity in neuronal cells. Neurotox Res. 2010; 17: 332-343. PubMed: https://pubmed.ncbi.nlm.nih.gov/19763737/
- Guo JJ, Liu YJ, Li MX, Yang YJ, Recker RR, et al. Linkage exclusion analysis of two candidate regions on chromosomes 7 and 11: leptin and UCP2/UCP3 are not QTLs for obesity in US Caucasians. Biochem Biophys Res Commn. 2005; 332: 602-608.
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commn. 1999; 257: 79-83. PubMed: https://pubmed.ncbi.nlm.nih.gov/10092513/
- Hotta, K, Funahashi T, Arita Y, Takahashi M, Matsuda M, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000; 20: 1595-1599. PubMed: https://pubmed.ncbi.nlm.nih.gov/10845877/
- Brand C, Gaya ACA, Dias AF, Agostinis-Sobrinho C, Farinha JB, et al. Relationship between insulin resistance and adipocytokines: the mediator role of adiposity in children. Annals of Human Biology. 2020; 47: 244-249. PubMed: https://pubmed.ncbi.nlm.nih.gov/32279531/
- Zhou M, Xu A, Tam PKH, Lam KSL, Huang B, et al. Upregulation of UCP2 by adiponectin: the involvement of mitochondrial superoxide and hnRNP K. PLoS One. 2012; 7: e32349. PubMed: https://pubmed.ncbi.nlm.nih.gov/22359684/
- Taghadomi Masoumi Z, Eshraghian MR, Hedayati M, Pishva H. Association between uncoupling protein 2, adiponectin and resting energy expenditure in obese women with normal and low resting energy expenditure. Gynecol Endocrinol. 2018; 34: 166-170. PubMed: https://pubmed.ncbi.nlm.nih.gov/29017362/
- Mahadik SR, Lele RD, Saranath D, Seth A, Parikh V. Uncoupling protein-2 (UCP2) gene expression in subcutaneous and omental adipose tissue of Asian Indians: relationship to adiponectin and parameters of metabolic syndrome. Adipocyte. 2012; 1: 101-107. PubMed: https://pubmed.ncbi.nlm.nih.gov/23700519/
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002; 415: 96-99. PubMed: https://pubmed.ncbi.nlm.nih.gov/11780125/
- Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci USA. 2014; 111: 960-965. PubMed: https://pubmed.ncbi.nlm.nih.gov/24395786/
- Pishva H. UCP2, SHBG, Leptin, and T3 Levels Are Associated with Resting Energy Expenditure in Obese Women. Endocr Metab Immune Disord Drug Targets. 2019.
- Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, et al. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J. 2008; 22: 9-18. PubMed: https://pubmed.ncbi.nlm.nih.gov/17855623/
- B Andrews, Z, Uncoupling protein-2 and the potential link between metabolism and longevity. Curr Aging Sci. 2010; 3: 102-112. PubMed: https://pubmed.ncbi.nlm.nih.gov/20158496/
- Cheng WC, Tsui YC, Ragusa S, Koelzer VH, Mina M, et al. Uncoupling protein 2 reprograms the tumor microenvironment to support the anti-tumor immune cycle. Nat Immunol. 2019; 20: 206-217. PubMed: https://pubmed.ncbi.nlm.nih.gov/30664764/
- Rask-Andersen M, Olszewski PK, Levine AS, Schiöth HB. Molecular mechanisms underlying anorexia nervosa: focus on human gene association studies and systems controlling food intake. Brain Res Rev. 2010; 62: 147-164. PubMed: https://pubmed.ncbi.nlm.nih.gov/19931559/
- Esteves P, Pecqueur C, Ransy C, Esnous C, Lenoir V, et al. Mitochondrial retrograde signaling mediated by UCP2 inhibits cancer cell proliferation and tumorigenesis. Cancer Res. 2014; 74: 3971-3982. PubMed: https://pubmed.ncbi.nlm.nih.gov/24853548/ B
- Schonfeld-Warden NA, Warden CH. Physiological effects of variants in human uncoupling proteins: UCP2 influences body-mass index. Biochem Soc Trans. 2001: 29: 777-784. PubMed: https://pubmed.ncbi.nlm.nih.gov/11709074/
- Mutombo B, Yamasaki M, Shiwaku K, UCP2 I/D modulated change in BMI during a lifestyle modification intervention study in Japanese subjects. Genet Test Mol Biomarkers. 2013; 17: 16-20. PubMed: https://pubmed.ncbi.nlm.nih.gov/23101559/
- Liu X, Zhang B, Liu X, Shen Y, Li J,, et al. A 45-bp insertion/deletion polymorphism in uncoupling protein 2 is not associated with obesity in a Chinese population. Biochem Genet. 2012; 50: 784-796. PubMed: https://pubmed.ncbi.nlm.nih.gov/22733179/
- Cortes-Oliveira C, Nicoletti CF, de Souza Pinhel MA, de Oliveira BAP, Quinhoneiro DCG, et al. UCP2 expression is associated with weight loss after hypocaloric diet intervention. Eur J Clin Nutr. 2017; 71: 402-406. PubMed: https://pubmed.ncbi.nlm.nih.gov/27759071/
- Kim YJ. A mixture of the aqueous extract of Garcinia cambogia, soy peptide and L: -carnitine reduces the accumulation of visceral fat mass in rats rendered obese by a high fat diet. Genes Nutr. 2008; 2: 353-358.
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010; 49: 1603-1616. PubMed: https://pubmed.ncbi.nlm.nih.gov/20840865/
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation. 2017; 114: 1752-1761. PubMed: https://pubmed.ncbi.nlm.nih.gov/15599400/
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-biological interactions. 2006; 160: 1-40. PubMed: https://pubmed.ncbi.nlm.nih.gov/16430879/
- Kizaki T, Suzuki K, Hitomi Y, Taniguchi N, Saitoh D, et al. Uncoupling protein 2 plays an important role in nitric oxide production of lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci. 2002; 99: 9392-9397. PubMed: https://pubmed.ncbi.nlm.nih.gov/12089332/
- Ryu JW, Hong KH, Maeng JH, Kim JB, Ko J, et al. Overexpression of uncoupling protein 2 in THP1 monocytes inhibits β2 integrin-mediated firm adhesion and transendothelial migration. Arteriosclerosis, thrombosis, and vascular biology. 2004; 24: 864-870. PubMed: https://pubmed.ncbi.nlm.nih.gov/15016641/
- Nègre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, et al. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997; 11: 809-815. PubMed: https://pubmed.ncbi.nlm.nih.gov/9271366/
- Marseglia L, Manti S, D'Angelo G, Nicotera A, Parisi E, et al. Oxidative stress in obesity: a critical component in human diseases. International journal of molecular sciences. 2015; 16: 378-400. PubMed: https://pubmed.ncbi.nlm.nih.gov/25548896/
- Huizing M, Ruitenbeek W, van den Heuvel LP, Dolce V, Iacobazzi V, et al. Human mitochondrial transmembrane metabolite carriers: tissue distribution and its implication for mitochondrial disorders. J Bioenerg Biomembr. 1998; 30: 277-284. PubMed: https://pubmed.ncbi.nlm.nih.gov/9733094/
- Cardel MI, Jastreboff AM, Kelly AS, Treatment of adolescent obesity in 2020. JAMA. 2019; 322: 1707-1708. PubMed: https://pubmed.ncbi.nlm.nih.gov/31566652/
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014; 384: 766-781. PubMed: https://pubmed.ncbi.nlm.nih.gov/24880830/
- Gadde KM, Martin CK, Berthoud HR, Heymsfield SB. Obesity. Pathophysiology and Management. 2018; 71: 69-84. PubMed: https://pubmed.ncbi.nlm.nih.gov/29301630/
- Tereshin, E, A role of fatty acids in the development of oxidative stress in aging. A hypothesis. Adv Gerontol. 2007; 20: 59-65. PubMed: https://pubmed.ncbi.nlm.nih.gov/17969588/
- Stępień M, Stępień A, Wlazeł RN, Paradowski M, Banach M, et al. Obesity indices and inflammatory markers in obese non-diabetic normo-and hypertensive patients: a comparative pilot study. Lipids Health Dis. 2014; 13: 29. PubMed: https://pubmed.ncbi.nlm.nih.gov/24507240/
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011; 29: 415-445. PubMed: https://pubmed.ncbi.nlm.nih.gov/21219177/
- Pihl E, Zilmer K, Kullisaar T, Kairane C, Mägi A, et al. Atherogenic inflammatory and oxidative stress markers in relation to overweight values in male former athletes. Int Jo Obes. 2006; 30: 141-146. PubMed: https://pubmed.ncbi.nlm.nih.gov/16158088/
- Vial G, Dubouchaud H, Couturier K, Cottet-Rousselle C, Taleux N, et al. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J Hepatol. 2011; 54: 348-356. PubMed: https://pubmed.ncbi.nlm.nih.gov/21109325/
- da Costa RM, Fais RS, Dechandt CRP, Louzada-Junior P, Alberici LC, et al. Increased mitochondrial ROS generation mediates the loss of the anti‐contractile effects of perivascular adipose tissue in high‐fat diet obese mice. Br J Pharmacol. 2017; 174: 3527-3541. PubMed: https://pubmed.ncbi.nlm.nih.gov/27930804/
- Xu H, Li F. miR127 aggravates myocardial failure by promoting the TGFbeta1/Smad3 signaling. Mol Med Rep. 2018; 18: 4839-4846. PubMed: https://pubmed.ncbi.nlm.nih.gov/30272299/
- Włodarczyk M, Nowicka G. Obesity, DNA damage, and development of obesity-related diseases. Int J Mole Sci. 2019; 20: 1146. PubMed: https://pubmed.ncbi.nlm.nih.gov/30845725/
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005; 115: 1111-1119. PubMed: https://pubmed.ncbi.nlm.nih.gov/15864338/
- Hanley AJ, Festa A, D’Agostino, Jr. RB, Wagenknecht LE, et al. Metabolic and inflammation variable clusters and prediction of type 2 diabetes: factor analysis using directly measured insulin sensitivity. Diabetes. 2004; 53: 1773-1781.
- Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. 2015; 13: 423-444. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4808277/
- Kim JD, Yoon NA, Jin S, Diano S. Microglial UCP2 mediates inflammation and obesity induced by high-fat feeding. Cell metabolism. 2019; 30: 952-962. PubMed: https://pubmed.ncbi.nlm.nih.gov/31495690/
- Jiang Z, Yang Q, Zhu C. UCP2 in early diagnosis and prognosis of sepsis. Eur Rev Med Pharmacol Sci. 2017; 21: 549-553. PubMed: https://pubmed.ncbi.nlm.nih.gov/28239813/
- McMurray F, Patten DA, Harper ME. Reactive oxygen species and oxidative stress in obesity—recent findings and empirical approaches. Obesity. 2016; 24: 2301-2310. PubMed: https://pubmed.ncbi.nlm.nih.gov/27804267/
- Mori S, Yoshizuka N, Takizawa M, Takema Y, Murase T, et al. Expression of uncoupling proteins in human skin and skin-derived cells. J Invest Dermatol. 2008; 128: 1894-1900. PubMed: https://pubmed.ncbi.nlm.nih.gov/18305572/
- Li W, Zhang C, Jackson K, Shen X, Jin R, et al. UCP2 knockout suppresses mouse skin carcinogenesis. Cancer Prevention Research. 2015; 8: 487-491. PubMed: https://pubmed.ncbi.nlm.nih.gov/25784177/
- Emre Y, Nubel T. Uncoupling protein UCP2: when mitochondrial activity meets immunity. FEBS Lett. 2010; 584: 1437-1442. PubMed: https://pubmed.ncbi.nlm.nih.gov/20227410/
Figures:
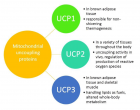
Figure 1
Figure 2

Figure 3
Figure 4
Similar Articles
-
The interaction between photonic technology and physical exercise: The action of low-level laser therapyAntonio Eduardo de Aquino Jr*,Fernanda Mansano Carbinatto. The interaction between photonic technology and physical exercise: The action of low-level laser therapy. . 2017 doi: 10.29328/journal.hodms.1001003; 1: 010-013
-
Behavioral factors of Abdominal Obesity and effects of lifestyle changes with Fiber AdequacyRoberto Carlos Burini*,Hugo Tadashi Kano,Mariana Santoro Nakagaki,Patricia Baston Frenhani,Katia Cristina Portero-McLellan. Behavioral factors of Abdominal Obesity and effects of lifestyle changes with Fiber Adequacy. . 2017 doi: 10.29328/journal.hodms.1001004; 1: 014-022
-
Upper gut bacterial overgrowth is a potential mechanism for Glucose Malabsorption after Vertical Sleeve GastrectomyTimothy R Koch*,Timothy R Shope,Matthew E Sharbaugh. Upper gut bacterial overgrowth is a potential mechanism for Glucose Malabsorption after Vertical Sleeve Gastrectomy. . 2017 doi: 10.29328/journal.hodms.1001006; 1: 030-035
-
Body mass index in a group of security forces (policemen). Cross-sectional studyGuillermo Padrón Arredondo*. Body mass index in a group of security forces (policemen). Cross-sectional study. . 2018 doi: 10.29328/journal.niogb.1001007; 2: 001-004
-
Herbal approach for obesity managementPreeti Singh*. Herbal approach for obesity management. . 2018 doi: 10.29328/journal.niogb.1001008; 2: 005-016
-
Dietary and Lifestyles assessment among Obese Women in Gaza City, PalestineMarwan O Jalambo*,Basil Kanoa,Mohammed S Ellulu,Smaher Younis,Mueen El-Kariri. Dietary and Lifestyles assessment among Obese Women in Gaza City, Palestine. . 2018 doi: 10.29328/journal.niogb.1001009; 2: 017-025
-
Obesity-Treatment by drugsSahithi G*. Obesity-Treatment by drugs. . 2019 doi: 10.29328/journal.niogb.1001010; 3: 001-001
-
ECHO…for a change!!Manish Motwani*,Rajeev Palvia,Bhavesh Nanda,Mahek Motwani,Bhakti Chaubal,Jyoti Kesarkar, Bhakti Mange,Sneha Shukla. ECHO…for a change!!. . 2020 doi: 10.29328/journal.niogb.1001011; 4: 001-003
-
Brown fat tissue: Therapeutic potential for insulin resistance, new hopes for tomorrowAlijani Sepideh*,Arefhosseini Seyedrafie. Brown fat tissue: Therapeutic potential for insulin resistance, new hopes for tomorrow. . 2020 doi: 10.29328/journal.niogb.1001014; 4: 022-023
-
The review of the relationship between UCP2 and obesity: Focusing on inflammatory-obesityMohamadreza Alivand*,Beitullah Alipour*,Sara Moradi,Yaser Khaje-Bishak,Maedeh Alipour. The review of the relationship between UCP2 and obesity: Focusing on inflammatory-obesity. . 2021 doi: 10.29328/journal.niogb.1001015; 5: 001-013
Recently Viewed
-
Endocrine abnormalities in two siblings with Rothmund Thomson SyndromeNagehan Aslan*,Ozgur Pirgon. Endocrine abnormalities in two siblings with Rothmund Thomson Syndrome. Ann Clin Endocrinol Metabol. 2018: doi: 10.29328/journal.acem.1001010; 2: 041-045
-
Obstructive Pyelonephritis Due to Postoperative Ureteral Stricture: A Case ReportAyoub Mamad*,Mohammed Amine Elafari,Mohammed Amine Bibat,Midaoui Moncef,Amine Slaoui,Tarik Karmouni,Abdelatif Koutani,Khalid Elkhader. Obstructive Pyelonephritis Due to Postoperative Ureteral Stricture: A Case Report. J Clin Med Exp Images. 2026: doi: 10.29328/journal.jcmei.1001039; 10: 003-005
-
Examining the Effects of High Poverty and Unemployment on Rural Urban Migration in Nigeria and its Consequences on Urban Resources and Rural DeclineTochukwu S Ezeudu*, Bilyaminu Tukur. Examining the Effects of High Poverty and Unemployment on Rural Urban Migration in Nigeria and its Consequences on Urban Resources and Rural Decline. J Child Adult Vaccines Immunol. 2024: doi: 10.29328/journal.jcavi.1001012; 8: 001-013
-
Case Report of a Child with Beta Thalassemia Major in a Tribal Region of IndiaNeha Chauhan, Prakash Narayan, Mahesh Narayan, Manisha Shukla*. Case Report of a Child with Beta Thalassemia Major in a Tribal Region of India. J Child Adult Vaccines Immunol. 2023: doi: 10.29328/journal.jcavi.1001011; 7: 005-007
-
Analysis and Control of a Glucose-insulin Dynamic ModelLakshmi N Sridhar*. Analysis and Control of a Glucose-insulin Dynamic Model. Ann Clin Endocrinol Metabol. 2026: doi: 10.29328/journal.acem.1001033; 10: 010-016
Most Viewed
-
Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trialSathit Niramitmahapanya*,Preeyapat Chattieng,Tiersidh Nasomphan,Korbtham Sathirakul. Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trial. Ann Clin Endocrinol Metabol. 2023 doi: 10.29328/journal.acem.1001026; 7: 00-007
-
Physical Performance in the Overweight/Obesity Children Evaluation and RehabilitationCristina Popescu, Mircea-Sebastian Șerbănescu, Gigi Calin*, Magdalena Rodica Trăistaru. Physical Performance in the Overweight/Obesity Children Evaluation and Rehabilitation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001030; 8: 004-012
-
Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging PresentationKarthik Baburaj*, Priya Thottiyil Nair, Abeed Hussain, Vimal MV. Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging Presentation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001029; 8: 001-003
-
Exceptional cancer responders: A zone-to-goDaniel Gandia,Cecilia Suárez*. Exceptional cancer responders: A zone-to-go. Arch Cancer Sci Ther. 2023 doi: 10.29328/journal.acst.1001033; 7: 001-002
-
The benefits of biochemical bone markersSek Aksaranugraha*. The benefits of biochemical bone markers. Int J Bone Marrow Res. 2020 doi: 10.29328/journal.ijbmr.1001013; 3: 027-031

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."