More Information
Submitted: December 21, 2020 | Approved: January 18, 2021 | Published: January 19, 2021
How to cite this article: Moradi S, Khaje-Bishak Y, Alipour M, Alivand M, Alipour B. Brown fat tissue: Therapeutic potential for insulin resistance, new hopes for tomorrow. New Insights Obes Gene Beyond. 2021; 5: 001-013.
DOI: 10.29328/journal.niogb.1001015
ORCiD: orcid.org/0000-0002-5847-3594
Copyright License: © 2021 Moradi S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: UCP2; Metabolism; Obesity; ROS; Inflammation
Abbreviations: IMM: Inner Mitochondrial Membrane; OXPHOS: Oxidative Phosphorylation; UCP: Uncoupling Protein; ROS: Reactive Oxygen Species; ETC: Electron Transport Chain; BAT: Brown Adipose Tissue; Ppars: Peroxisomal Proliferators-Activated Receptors; TRE: Thyroid Hormone Response Elements; RXR: Retinoid X Receptor; SREBP: Sterol Regulatory Element Binding Protein; FFA: Free Fatty-Acid; BMI: Body Mass Index; POMC: Proopiomelanocortin; MS: Multiple Sclerosis; DM: Diabetes Melitus; Mes: Metabolic Syndrome; RA: Rheumatoid Arthritis; SLE: Systemic Lupus Erythematosus; NTD: Neural Tube Defects; PCOS: Polycystic Ovary Syndrome
The review of the relationship between UCP2 and obesity: Focusing on inflammatory-obesity
Sara Moradi1, Yaser Khaje-Bishak2, Maedeh Alipour3, Mohamadreza Alivand4* and Beitullah Alipour5*
1Student`s Research Committee, Department of Nutrition, Tabriz University of Medical Science, Tabriz, Iran
2Department of Nutrition, Maragheh University of Medical Sciences, Maragheh, Iran
3Medical Student, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Medical Genetics, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
5Department of Community Nutrition, Faculty of Nutrition, Tabriz University of Medical Sciences, Tabriz, Iran
*Address for Correspondence: Beitullah Alipour, Department of Community Nutrition, Faculty of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran, Tel: (+98-413) 3357581; Fax: (+98-413) 3340634; Email: [email protected]
Mohamadreza Alivand, Tabriz University of Medical Sciences, Faculty of Medicine, Iran
Obesity is rising worldwide, and the inflammatory disease increased in parallel. Many studies demonstrate excess fat mass is an indicator of obesity. As much as lipid increased in the cell, ROS production increased. On the other hand, ROS could enhance lipid storage and increased adiposity. So obesity and inflammation have a reciprocal relationship. Uncoupling protein2 (UCP2) could control the metabolism of energy, adipose tissue, and weight management. Also, UCP2 decreased ROS, oxidative stress, and inflammation. Therefore, as metabolism-related to oxidative stress and inflammatory status, and by considering the modulatory contribution of UCP2 in inflammation; it seems UCP2 could link obesity and inflammation. This study aims to review the studies about the association between UCP2 and obesity focusing on the inflammatory process linked to ROS. In conclusion, as the results contradict the association between UCP2 as the center of metabolism and obesity, obesity-related hormones, and oxidative stress, further studies in human trials are recommended.
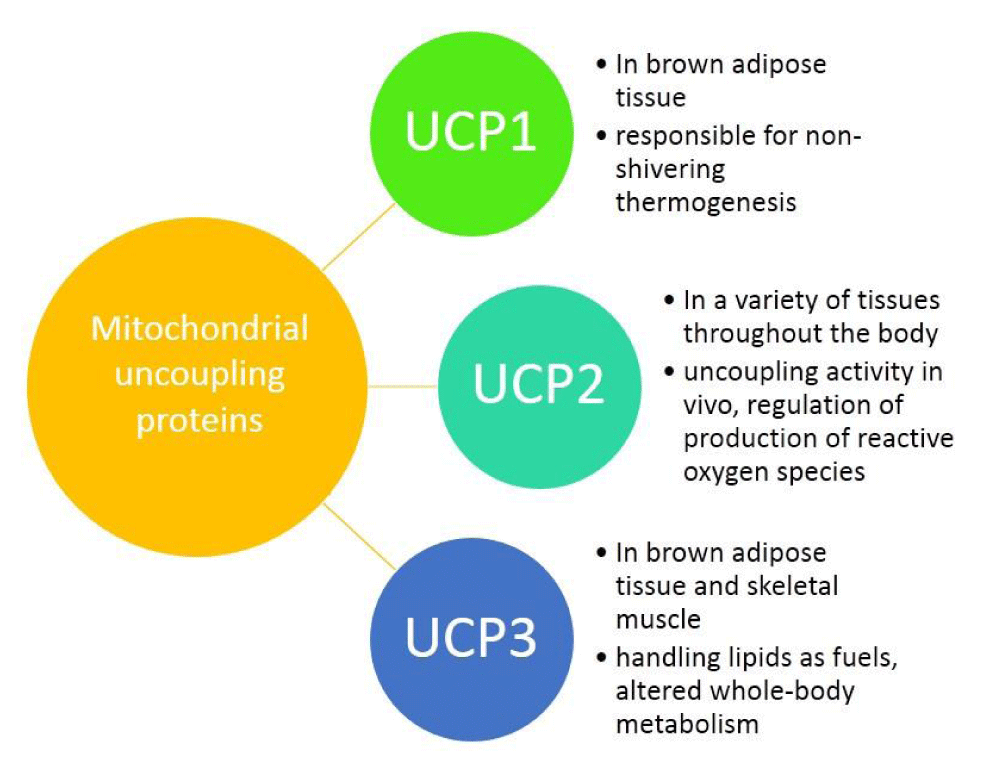
Understanding the obesity-related genes may provide future therapeutic strategies to modulate disease progression. UCP2 separates oxidative phosphorylation (OXPHOS) from ATP production in the inner mitochondria. Figure 1 shows the differences among UCP1, 2, 3. The main role of UCP2 is controlling the metabolism of energy in the cells [1-3]. Besides that, the expression of UCP2 is associated with chronic inflammation due to reactive oxygen species (ROS). In this regard, in injured cells and tissues, ROS could be decreased by reducing the proton motor force by the anti-inflammatory effect of UCP2 [4].
Figure 1: The differences among UCP1, 2, 3.
Different pieces of evidence show a bilateral association between obesity and inflammation [5-10]. As obesity is a chronic inflammatory condition it is modulated by inflammatory-related biomarkers, microRNAs, hormones, and cytokines [11-13]. In this regard, ROS, as a marker of inflammation, could trigger lipid production and enhanced adiposity. On the other hand, obesity increased inflammation in different ways, for example, overfeeding produces more ROS; increases oxidative stress-related adipokines; and decreases anti-oxidant potentials that lead to the development of obesity-related complications like some cancers [14-17].
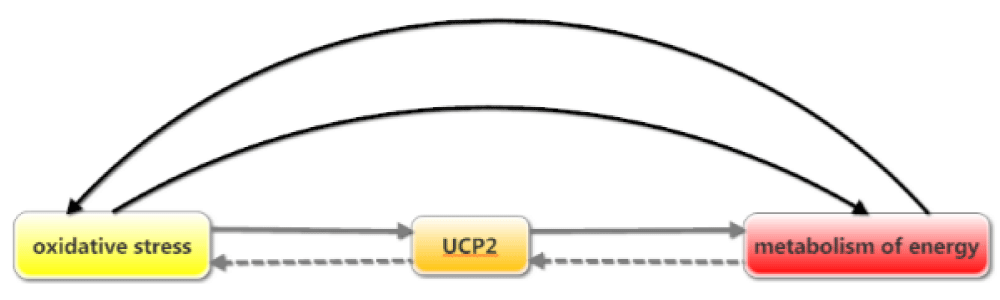
As UCP2 contributes to metabolic functions it could be associated with indicators of energy metabolism, adiposity, body weight, BMI, body composition, fat mass, and lean body mass [18,19]. Therefore, as cell metabolism-related to oxidative stress and the inflammatory status of cells, and by considering the modulatory role of UCP2 in inflammation; it seems UCP2 is a master protein in the connection between obesity and inflammation [20]. Figure 2 demonstrate the relationship between these three elements: UCP2, energy metabolism, and oxidative stress. So UCP2 might have a substantial role in the onset, progress, or treatment of obesity. This study aims to review the studies about the association between UCP2 and obesity focusing on the inflammatory process linked to ROS.
Figure 2: Relations between oxidative stress, UCP2, and metabolism of energy.
UCP2
Recent studies show that UCPs are found in eukaryotes such as plants, fishes, and mammals. UCPs are proteins that separate OXPHOS from ATP synthesis. In mitochondria, the electron transport chain (ETC) is in charge of ATP production via an electrochemical gradient across the IMM. UCPs bypass this gradient by induced proton leak, so they waste energy as heat and reduced ATP yield and prevent the storage of energy as fat mass [21,22]. The UCP1 was discovered in the brown adipose tissue (BAT), UCP2 is expressed in many tissues and UCP3 is expressed in the skeletal muscle [23-25]. Additionally, UCP2 and UCP3 show near 60% sequence similarity with UCP1 and near 70% similarity with each other. This similar identity probably makes a similarity in their biochemical functions.
The main physiological function of UCPs is controlling the body’s metabolism and modulate the ROS [23,26,27]. UCP1 in rodents is the main regulator of diet- and cold-induced thermogenesis. So it could control the energy expenditure and the energy balance in the body [28-30]. Nonetheless, adult humans only have little amounts of BAT, so the physiological significance of UCP1 for them has been debatable [31,32]. Although, some studies showed UCP2 has not rolled in adaptive thermogenesis or regulation of body metabolism [33,34].
Generally, UCP2 is expressed in the spleen, lung, intestine, skin, brain, fibroblasts, immune cells, kidney, pancreatic islets, skeletal muscle, heart, and adipose tissue [26,35-37]. Under the basal condition, the turnover rate of UCP2 is around 3 days that is parallel to UCP1 and in different situations, in response to changes in nutrient supply, the turnover rate of UCP2 was different. Besides it was showed that the half-life of UCP2 is short and it was about less than 1 hour [35], so it controls energy needs in the short term.
Nonetheless, the properties of UCP2 differ between populations. One explanation for this difference is the existence of genetic variants that make various UCP2 characteristics and multiple levels of function. For example, in human studies, it was shown that polymorphism of the UCP2 makes different phenotypes. In -866G/A polymorphism for the promoter of UCP2, the G allele is associated with a lower expression of UCP2 when compared to the A allele [38] and this polymorphism modulates the development of diabetes [39]. In another study of Egyptian children and their mothers, GG genotype after AG was the most frequent and the G allele was the most present in mothers who affected with obesity and male children who also have obesity, (statistical significance was not observed) [37].
Regulation of UCP2 in the cells
UCP2 is regulated at multiple levels including transcription, translation, protein activity, and turnover, and activity. These regulations take place by the effect of hormones, cytokines, and neurotransmitters. Especially, the transcription of the UCP2 is regulated by factors such as fatty acids and amino acids, glucose, and glutamine [40]. In this regard, polyunsaturated fatty acids have a definite role [41]. Fatty acids enhance UCP2 gene expression through peroxisomal proliferator-activated receptors (PPARs) [42]. Similarly, the agonist of PPAR-γ increased UCP2 expression in adipocytes [43]. Additionally, nuclear receptors such as sterol regulatory element-binding protein (SREBP), retinoid X receptor (RXR), and thyroid hormone response elements (TRE); contribute to UCP2 expression. For example, PPAR-γ in complex with RXR, by binding to the intron 1 of the UCP3 gene, which is located near UCP2, simplifies the translational activation of UCP2 [44]. In another example, PPAR-γ coactivator1-a (PGC1a) has increased UCP2 gene expression in animal models of type II diabetes in the pancreatic beta-cell [45-47].
Despite in translation level, the mRNA of UCP2 is constantly suppressed. In cells such as macrophages, the level of UCP2 protein was altered despite unchanged mRNA expression that suggests post-transcriptional regulation [48]. So once UCP2 is translated, its activity can also be regulated. It was found polyunsaturated fatty acids, can increase the activity of UCP2. Other substrates might be regulated UCP2 activity, even ROS-derived lipid peroxidation products such as 4-hydroxynonenal, has been found to increase the activity of UCP2 in IMM and promote proton leak [3]. Although generally, the regulation mechanism of UCP2 activity by ROS is not clear. However, in a normal redox state, ROS levels are maintained at tolerable levels in mitochondria, so UCP2 is conjugated with glutathione (GSH) which is an inactive form. When small increases in ROS levels occur, UCP2 is deglutathionylated, resulting in an increased proton leak [49].
Regulation of UCP2 occurs in different ways depending on different tissues and health or disease status. This level of regulation depends on the kind of tissue (adipose tissue, skeletal muscles, and brain neurons), as the most important issue here is the adipose tissue. Adiponectin, an adipocyte-derived cytokine, can increase UCP2 expression that relates UCP2 with adiposity. Irisin, an ischemic skeletal muscle-derived cytokine, up-regulates UCP2 expression in the lung [50]. In the CNS, ROS production in proopiomelanocortin (POMC) and neuropeptide Y (NPY)/agouti-related peptide (AgRP) regulates UCP2 expression [51,52], these regulators suggest the probability of the incorporation of UCP2 in appetite control that effects behavioral eating, cachexia, and obesity.
The difference between the expression of UCP2 in tissues such as the heart and skeletal muscle also suggests a potential regulatory role of microRNA. Some miRNAs repress or enhance UCP2 gene expression. MicroRNA-133a [53] and 15a [54] enhance UCP2 gene expression and it down-regulated by miR-24 and miR-34a, [55]. These regulations were very complicated. A muscle-specific miRNA, miR-133a recognizes the 3′-UTR of mouse UCP2 mRNA and binds to upstream of miR-133a and suppressed UCP2, through the synthesis of muscles [56,57]. This increased myogenesis so that might be decreased adiposity.
Multiple mechanisms can control the level of activity of UCP2, including a variety of metabolites. It was not extended studied exist, but few food items were showed affected body metabolism that might be through UCP2. For example, Kim, et al. showed green satsuma mandarin orange extract (GME) induced UCP2 expression in skeletal muscle. Besides, Muhammad, et al. demonstrated that in participants with AA + GA genotypes of UCP2, more coffee intake decreased the weight, BMI, and fat mass, but in GG genotype there was no association with body composition observed [58].
The act of UCP2 in related cells
The precise function of UCP2 in cells remains unknown, although UCP2 is involved in a variety of physiological and pathological procedures. Many studies showed UCP2 acts in the center of cell metabolism [59-62]. As before noted UCP2 has three main roles in the body. Two of them are related to energy metabolism and thermogenesis. The third role corresponds to oxidative stress and inflammation.
UCP2 and energy metabolism
The well-known act of UCP2 is controlling body metabolism by modulates ATP production and proton leak in IMM, so it wastes energy in the form of heat and regulates cold- and diet-induced thermogenesis [63-66].
UCP2 in CNS energy homeostasis
In the central nervous system (CNS) UCP2 regulates homeostatic mechanisms. As the second function, UCP2 affects food intake, appetite, energy consumption, nutrients homeostasis, reward behaviors, secretion of metabolism-related hormones; and negative regulation of glucose sensing systems. Central UCP2 affects these processes by the mechanisms that are related to the histone deacetylation, synaptic, and mitochondrial processes [4,67-69]. Besides these impacts, UCP2 is highly expressed in the hypothalamus, specifically in the arcuate and ventromedial nuclei [51], which contains POMC and NPY/AgRP, the called anorexigenic and orexigenic neurons [51,70-72], by this process, UCP2 could be linked with appetite. Until now no study evaluates this relationship.
UCP2 in the peripheral energy homeostasis
UCP2 controls nutrient homeostasis through hormonal regulations. In beta cells of the pancreases, UCP2 is involved in glucose-stimulated insulin secretion (GSIS). Increased levels of glucose enhanced ATP/ADP ratio in the mitochondria. The increased ATP inhibits ATP-sensitive potassium channels, caused increased plasma membrane depolarization, and enhanced insulin secretion [73]. Through this process ucp2 expression is enhanced. In addition to insulin, UCP2 has also been reported to regulate glucagon secretion from pancreatic alpha cells. The absence of UCP2 in alpha cells by enhanced mitochondrial coupling makes more ROS in the cells that suppress glucagon secretion. Considering the main role of these two hormones in energy homeostasis and by considering the importance of UCP2 in their secretion, it represents a sensor in the regulation of hyperglycemia [74]. Another metabolic hormone related to UCP2 is ghrelin. It has been hypothesized that fasting enhanced ghrelin secretion that induced UCP2 activation. This effect is mediated by increased oxidation of fatty acids that elevate the ROS levels [75]. Prevention of ROS production in POMC neurons during diet-induced obesity, activate UCP2 that impairs the activity of these neurons during hyperglycemia, so leptin resistance occurred, in which elevated levels of leptin do not reduce in feeding or increase energy expenditure [76]. These hormones have affirmed the association of UCP2 with metabolism-related diseases like obesity and diabetes.
UCP2 in adipose tissue
Although lean body mass is an important organ for the use of energy and protection against fat storage, the expression and function of UCP2 in lean body mass have remained unclear, but many studies show the association of fat mass (in the form of adipose tissue) and UCP2 expression. According to these studies, human adipose tissue is divided into brown and white adipose tissue. The brown adipose tissue is responsible for the thermogenesis in the body, and white adipose tissue is responsible for fat storage. White adipose tissue with different types of cells such as fibroblasts, pre-adipocytes, mature adipocytes, and macrophages [72,77-79]. Furthermore, visceral fat mass has been considered an energy storage location and an endocrine organ to release adipocytokines [80]. Some of these adipokines like adiponectin unregulated UCP2 expression.
White adipose tissue release leptin that acts on the hypothalamic regions of the brain which maintains body metabolism, regulates energy homeostasis, decreasing energy intake, and increasing energy expenditure circulates at levels proportional to the amount of adipose tissue, signaling long-term energy storage [81,82]. The study of Scarpace, et al. indicates that leptin increases the gene expression of UCP2 [83]. Ho, et al. showed leptin may preserve neuronal survival via UCP2 by keeping the ATP levels and the membrane potential of mitochondria, but leptin had no effect in the adjustment of ROS levels [84]. Unless in the US Caucasians affected with obesity, leptin having an effect on fat mass at effect sizes of 5% or greater, and UCP2/UCP3 have the effect size of 10% or greater, they unlikely to have a substantial effect on variation in obesity phenotypes [85].
Decreased adiponectin from adipocytes plays an important role in obesity-related diseases [86,87] due to insulin-sensitizing, anti-inflammatory effects, and decreases body weight [88]. Zhou, et al. suggest adiponectin stimulates Mitochondrial superoxide production that promotes UCP2 expressions in mice liver [89]. It was showed in women with obesity UCP2 protein has a significant relationship with plasma adiponectin [90]. In addition, Mahadik, et al. demonstrate as for UCP2 gene expression was significantly reduced in patients with obesity, a relationship between adiponectin and UCP2 expression may provide us with an innovative therapeutic strategy to prevent obesity-related diseases [91]. These studies provide growing evidence that shows the critical role of adipocyte released-cytokines in UCP2 expression and vice versa.
Studies showed UCP2 through thermogenesis increased utilization of energy in the body, so decreased energy storage as fat accumulation [92]. On the other view, Vozza, et al. showed UCP2 transport malate, oxaloacetate, and aspartate [93], which suggest an additional role for UCP2 in nutrient metabolism, especially fatty acids [94]. In addition to proton leak and waste of energy, UCP2 controls lipid metabolism. Cholesterol side-chain cleavage enzyme (CYP11A1) is related to UCP-2 expression, which demonstrated the contributing role of UCP-2 in lipid metabolism [25]. Moreover, UCP2 has a role in mitochondrial utilization of fatty acids and pyruvate [95], so it could modulate the intracellular usage of nutrients. On the other hand, UCP2 prevents the overproduction of ATP [96].
The relation of UCP2 with obesity and its comorbidities
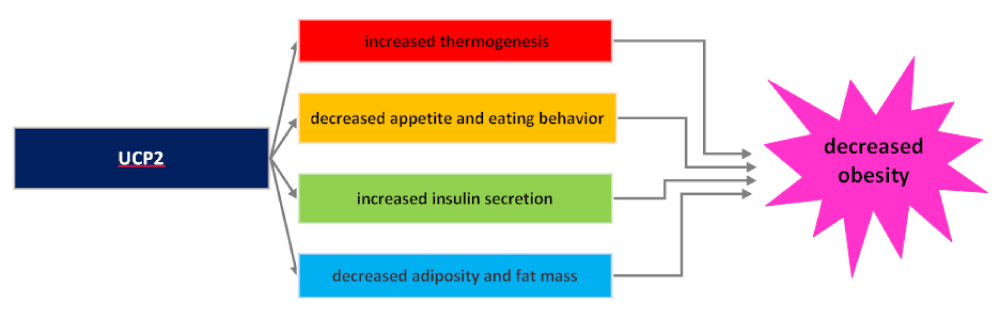
Because UCP2 is located in the center of energy metabolism, it plays an important part in the onset, diagnosis, and treatment of obesity. In the onset phase, UCP2 through heat generation regulates thermogenesis and energy consumption which prevents adipose tissue accumulation [62]. Figure 3 demonstrate the mechanisms for decreased obesity by UCP2.
Figure 3: The mechanisms by which UCP2 decreased obesity.
UCP2 could be used as a diagnostic tool for obesity [97]. Some diseases such as obesity and its comorbidities (PCOS, diabetes, etc.) display a probable association between UCP2 and the grade of disease [98]. For example, the expression of UCP2 in tumor cells determines the features of the tumor microenvironment and is positively associated with prolonged survival. These results could be influenced by different phenotypes [97,99]. In PCOS patients the correlation between UCP2 and CYP11A1 in lipid metabolism could be used as a diagnostic tool for obesity [25]. As WHO demonstrated UCP2 is an applicable tool for evaluating obesity grade but, studies in humans have produced only weak evidence for the association of variants of UCP2 with BMI because genetic variations could change the results [100]. In Japan, Mutombo, et al. found UCP2 D/I associated with weight by altering the effect of energy expenditure on BMI [101], but in the Chinese population, no association was demonstrated between UCP2-45 bp I/D and BMI variation [102]. Although a higher UCP2 expression could have a negative relationship with the stage of obesity, there was controversy here; Cortes-Oliveira C, et al. showed UCP2 expression contributed to weight loss after hypocaloric diet intervention in animals [103]; and in the study of Pishva, et al. increased UCP2 related with decreased the REE level in women affected by obesity [94], and as showed many years ago, more decreased in energy expenditure (as REE) should be related with more fat storage and overweight; so, in women affected with obesity enhanced UCP2 related with enhanced obesity. As oxidative stress indicators act like obesity indicators, UCP2 could be a diagnosis tool for obesity, but further studies need to determine the exact amount of UCP2 expression in different tissues related to the grade of obesity as a biomarker.
For the treatment of obesity, control of UCP2 might be useful. In patients with obese cell’s energy metabolism changes from consumption of fat to the storage of fat. Because UCP2 prevents this mechanism and reverse this phenomenon, it may offer a practical anti-obesity strategy. With this aim, in some studies, treatment of disease with control metabolism-related genes was investigated and it was suggested that UCP2 could be a molecular target for curing obesity and its complications [104]. Additionally, unless the mechanism is unknown, the ability of UCP2 to reduce oxidative stress turns it into an attractive therapeutic target in obesity and its comorbidities, in which ROS has a key role in their pathogenesis. More trials should be done for the final conclusion.
Mutual association of UCP2 and oxidative stress
Oxidative stress is a normal phenomenon in the body. Under normal conditions, the levels of ROS in the cells are maintained at low levels. Besides, oxidative stress is a disequilibrium between the pro-oxidants and antioxidants in the body. ROS and nitrogen species could be biomarkers for oxidative damage. Every single cell in the body tends to establish stable conditions between oxidative and antioxidant species. The continuous formation of ROS and other free radicals is important for normal physiological functions such as catabolic and anabolic processes. However, endogenous biologic factors or exogenous environmental factors, such as radiation excess the produce of free radicals. Mitochondria is the most important location of contentious cellular ROS production due to the electron transport chain in the mitochondrial membrane. ROS has several physiological roles such as cell signaling, therefore causing the imbalance that leads to cell and tissue damage [105-107].
The third role of UCP2 that was recently more noticed is modifying oxidative stress in cells [26]. It was shown that the B-oxidation of fatty acids produced a greater number of electrons and increases ETC activity more than other substrates, so it promotes more ROS. Superoxide, a lipid peroxidation product and a frequent ROS in the cell could activate UCP2, facilitated proton leak in IMM, controlled proton re-entry into the mitochondrial matrix thus reduced the ROS re-production [96]. Besides proton transfer, UCP2 allows the passage of C4 metabolites that substrates the Krebs cycle and therefore decreases the activity of ETC, ATP yield, and ROS production [93].
As many studies showed, UCP2 controls body oxidative stress in a feedback manner and there is a bilateral relationship between UCP2 and ROS. For example, in macrophages overexpression of UCP2 decreased intracellular ROS levels and reduced immune activity [108-110], and also, in immune cells, overproduction of ROS increased expression of UCP2 [111,112]. Because of UCP2 relationship with ROS as an indicator of oxidative stress, Echtay, et al. suggested UCP2 could be acted as a sensor of oxidative stress and it is the critical protein for modulates ROS within the cell [3].
Obesity
Overweight and obesity present the most challenging chronic disease to prevention around the world. The American Medical Association demonstrates obesity as a disorder and considers it one of the main public health issues [113]. Overweight and obesity are defined as increased fat accumulation that may disturb health and it is more fatal than underweight. In 2016, 39% of adults were overweight and 13% were people with obesity [113]. Obesity is the main risk factor for metabolism-related diseases such as cardiovascular diseases, diabetes, osteoarthritis, and colon cancers [113].
Obesity is a multi-caused disease that has complex pathogenesis, with environmental, genetic, and epigenetic factors [114]. Obesity that generated by the gradual accumulation or dysfunction of adipose tissue, the abnormal or excessive fat, that may interfere with the maintenance of an optimal state of health [115]. This tissue is considered an endocrine organ with high lipid storage capacity for systemic management of energy substrates. Evidence showed excessive fat accumulation in individuals with obesity, induced to a pathological increment of FFA levels in serum which impairs the metabolism of energy substrates such as fat and glucose, adipose tissue, and promotes higher mitochondrial and peroxisomal oxidation witch causes synthesis of free radicals, oxidative stress, depletion of ATP, and lipotoxicity [116].
Obesity and inflammation
Inflammation is a series of events that occur to preserve tissue and organ stability. Releasing the mediators and expressing the receptors at the appropriate time is necessary to regenerate and main the tissue. Additionally, inflammation is a protective function of tissue for the response to destruction [117], so it may rebuild tissue. Obesity is associated with a chronic low-grade inflammation in which pro-inflammatory cytokines increased. Although the triggers were not clear. A possible hypothesis is that in adipocytes the over-expression of genes induces intracellular stress, resulting in the activation of inflammatory cascades, is accrued [118]. Moreover, the sensitivity of oxidative biomarkers is higher in individuals who have been affected by obesity and associated directly with BMI, percentage of body fat, LDL oxidation, and triglyceride levels [119]. Furthermore, in addition to the increase of adipose tissue, the activity of antioxidants such as superoxide dismutase, catalase, and glutathione peroxidase, significantly diminished, which could increase levels of ROS [36].
There is a two-sided association between ROS and adiposity. In two animal studies to explore the role of ROS in obesity, it was found that excessive fat accumulation was associated with increased ROS, and also it was demonstrated that more ROS production can cause more fat accumulation and change in the fatty acids composition [120, 121]. On other words, increased ROS contributes to the development of adiposity by enhanced production of obesity-related cytokines.
Excessive lipid storage produces stress in adipocytes with the ability to release pro-inflammatory products. In the other hand, in pathological situations, adipose tissue, adipocytes, and pre-adipocytes secret bioactive molecules called adipokines, including cytokines (TNF-α, IL-1, and IL-6), hormones (leptin and adiponectin), and ROS [111]. In the positive feedback, these cytokines are potential triggers for the production of ROS and nitrogen by macrophages and monocytes; therefore, a rise in the number of cytokines could be responsible for increased inflammation. Adipose tissue’s innate immune system affected inflammation due to increased pro-inflammation, trigger the acute phase response, and promote oxidative stress. So, a study suggested that inflammation in adipose tissue of patients with obesity plays a critical role in the pathogenesis of obesity-related comorbidities [122]. Besides these findings, obesity alters genome constancy. Oxidative stress usually occurred in adipocytes that could damage DNA and inhibit its repair. On the other hand, the accumulation of damaged DNA can cause enhanced mutation and change the gene expression that make disturbances in cell metabolism, proliferation and migration, and resistance to apoptosis [123]. All of these related the obesity and inflammation through DNA.
Furthermore, visceral adiposity is associated with the more prevalence of metabolic disorders such as diabetes mellitus (DM), hypertension, cardiovascular disease, and other chronic diseases, all of which coincide with degrees of inflammation [124,125]. There is piece of of evidence suggesting obesity is a chronic low-grade inflammation disease, so oxidative stress, in particular, the imbalance of ROS may be the mechanistic link between obesity and its associated cardiovascular and metabolic complications [126].
Several things can cause chronic inflammation, such as infection, injury, an autoimmune disorder, long-term exposure to irritants. Although, some of them mistakenly attacking healthy tissue and are related to ROS production. One of the most important factors to consider in the adjustment of ROS production is UCP2. In recent years, there have been significant advancements in our understanding of how UCP2 contributes to the start and continue of inflammation [56,127,128].
The association of UCP2 and inflammation in obesity
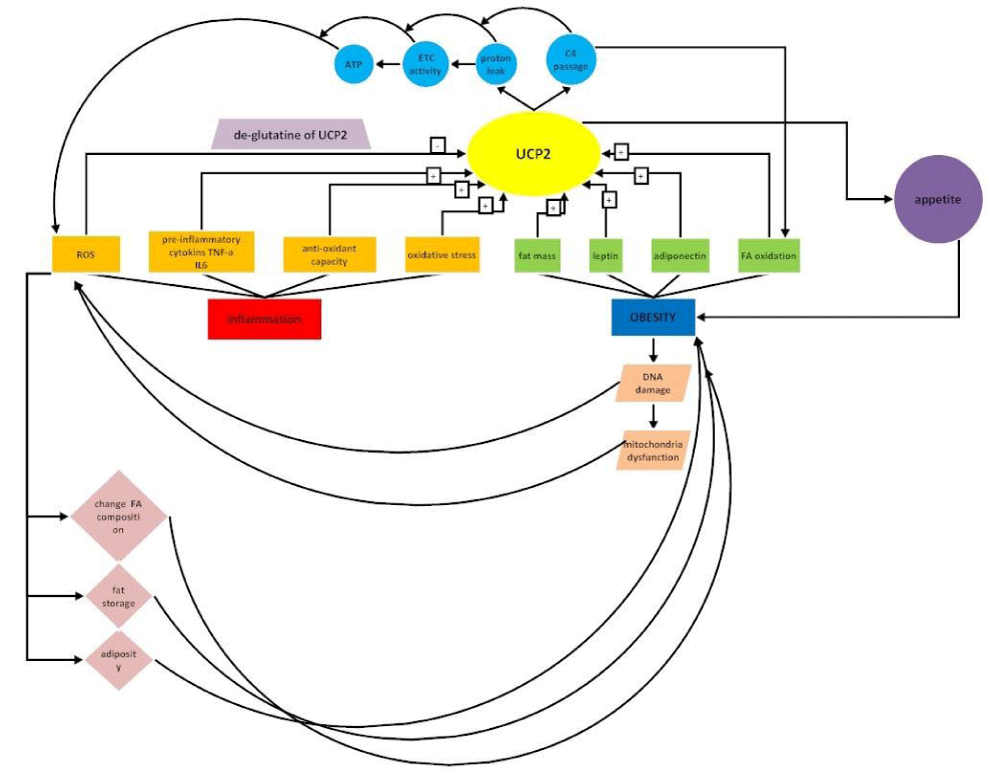
While intracellular ROS production takes place in many cells, mitochondrial production of ROS remains a part of inflammation in most cells [129]. There is evidence that UCP2 has the main role in maintaining steady-state levels of ROS. For example, the skin is frequently exposed to various chemical, physical, and biological stresses, such as microbial infection, ultraviolet rays, and temperature changes, which enhanced the skin secretions, peroxidation of lipids, and degeneration of proteins with inflammatory process or immune response. It was shown, UCP2 in the skin may be involved in regulating the production of skin ROS by controlling via the b-adrenergic receptor and retinoid receptor[130,131]. Studies demonstrated UCP2 -866G allele is correlated with lower levels of UCP2 expression in the immune systems. The relation of UCP2 with the inflammatory disease was suggested in many studies. Many evidences highlight the role of UCP2 in a broad range of normal and un-normal processes [132]. Studies suggest some chronic diseases have inflammatory nature and in the others after the onset of disease, oxidative stress increased and inflammatory status developed. Table 1 shows some of these diseases. Figure 4 demonstrates the overall role of UCP2 in inflammatory-obesity.
| Table 1: The role of UCP2 in inflammatory disease. | ||||||
| Disease | Results | The Pathologic Mechanism of low expression of UCP2 | Ref. | Year | ||
| Increased ROS | Decreased thermogenesis | Decreased ATP production | ||||
| PCOS | The increase in expression of ovary UCP2 identified when treated with T3. | * | (1) | 2011 | ||
| –866 G/A polymorphism of theUCP2 is not associated with the pathogenesis of PCOS. | * | (2) | 2011 | |||
| RA, SLE | –866 G/A polymorphism of theUCP2has a protective role in chronic inflammatory diseases. | * | (3) | 2009 | ||
| MS | The increase inUCP2expressioncorrelated with an augmented number of CD3+T-lymphocytes that regulate ROS in MS. | * | (4) | 2017 | ||
| No significant differences were revealed in the frequencies of alleles at –866 G/A polymorphism of theUCP2 (Article in Russian). | * | (5) | 2011 | |||
| Results confirm the link betweenUCP2SNP and MS in a Spanish MS population. | * | (6) | 2007 | |||
| –866 G/A polymorphism of theUCP2is associated with susceptibility to MS in the German population. | * | (7) | 2005 | |||
| Cancer | The overall survival probability of lung carcinoma patients with high mRNA expression levels of UCP2 and PRMT1 is strongly reduced. | * | (8) | 2017 | ||
| In lung cancer cell lines exposed to hypoxia or oxidative stress while Ucp2 showed the modest up-regulation in stress conditions. | (9) | 2017 | ||||
| Genotypes of UCP2rs659366 were not associated with colorectal cancer risk but the interactions ofUCP2rs659366 and red meat consumption may contribute to the risk of colorectal cancer. | * | (10) | 2013 | |||
| UCP2 expression is increased in most human colon cancers, and the level of expression appears to correlate with the degree of neoplastic changes. | * | (11) | 2004 | |||
| MeS | The 45-bp I/D polymorphism of UCP2 was associated with decreased risk of MeS. | * | * | (12) | 2014 | |
| UCP2 gene expression was reduced in MeS patients compared with controls. | * | (13) | 2012 | |||
| DM | UCP2gene may be involved in the pathogenesis ofDiabetic Retinopathy in Han Chinese Patients with Type 2Diabetes. | * | (14) | 2018 | ||
| The UCP2 haplotype Ala55Val (rs660339) seems to be an important risk factor associated with proliferative diabetic retinopathy in both type 2 and 1 diabetic groups. | * | (15) | 2010 | |||
| NAFLD | UCP2 show wide tissue distribution with a substantially increased presence in fatty liver. | * | (16) | 2005 | ||
| CVD | The G allele ofUCP2rs2735572 and T allele ofUCP2rs17132534 were associated with higher diastolic blood pressurethat was associated with a higherCVDrisk factors. These findings suggest that UCP2 may have a role in the development ofCVD. | * | (17) | 2020 | ||
| –866 G/A polymorphism of the UCP2 occurred at highest frequency in CAD patients, but –866 G/A polymorphism of the UCP2 did not influence the risk of CAD in South African Indian. | (18) | 2013 | ||||
| UCP2 SNPs were associated the totalCVD, MI, and ischemic stroke risk. | * | (19) | 2011 | |||
| Obesity | –866 G/A polymorphism of the UCP2 may play a crucial role in the pathogenesis of insulin secretion thus leads to the development of DM. | * | (20) | 2019 | ||
| The differences of UCP2mRNA expression level between the obese individuals and the controls as well as between the DM patients and the controls did not reach statistical significance. | * | (21) | 2017 | |||
| An association between adiponectin and UCP2 gene expression may provide therapeutic strategy to prevent obesity. | (13) | 2012 | ||||
| –866 G/A polymorphism of the UCP2 in the Iranian population, Subjects with AA genotypes in all of the studied groups showed a lower BMI than subjects with the GG genotype. | (22) | 2010 | ||||
| AUCP2gene exon 8 variant that may affect susceptibility to weight gain by influencing regulation of leptin. Also UCP2raised body mass index. | * | (23) | 1999 | |||
| UCP2 polymorphism was shown to be associated with energy metabolism and obesity in humans. DNA sequencing of UCP2 revealed polymorphisms Ala→Val substitution in exon 4 and 45 bp insertion/deletion in the 3′-untranslated region of exon 8 ofUCP2 that contributed to a variation in metabolic rate and overall body fat content. | (24) | 1998 | ||||
CAD: coronary-artery disease, CVD: cardiovascular disease, DM: diabetes mellitus, MeS: metabolic syndrome, MS: multiple sclerosis, NAFLD: non-alcoholic fatty liver disease, PCOS: polycystic ovary syndrome, PRMT1: protein arginine methyltransferase 1, RA: rheumatoid arthritis, ROS: reactive oxygen species, SLE: systemic lupus erythematosus, UCP: uncoupling protein.
|
||||||
Figure 4: The overall role of UCP2 in inflammatory-obesity.
Understanding these mechanisms will be a key factor to explain the protective effects of UCP2 in therapies for various diseases. Because UCP2 synthesis is regulated by miRNAs, nutrients, cytokines, and hormones; and it was shown UCP2 itself, has a regulatory effect with these factors of control in a feedback manner. Every change in these controlling factors at each level could change UCP2 and create or treat disease. Studies showed that energy and metabolism-related diseases such as obesity, associated with genetic status. As UCP2 has a role in metabolic functions, it is considered an important regulatory indicator of body metabolism, body weight, and body composition. As UCP2, has a role in oxidative stress by ROS modulation, it participates in the etiology or progression of inflammatory diseases such as obesity and its comorbidities.
Although a higher UCP2 expression could have a negative relationship with the stage of obesity, findings are controversial; as UCP2 expression contributed to weight loss in animals, decreased the REE in humans, related with leptin and adiponectin. Further studies are recommended to determine the mechanism of action. In conclusion, UCP2 could be used as a diagnostic tool for inflammatory obesity. Additionally, without considering the mechanisms, the ability of UCP2, to reduce oxidative stress makes it an attractive therapeutic goal in obesity, in which ROS production plays a key role in pathogenesis and treatment. More studies should be done for the final conclusion.
Funding
All parts of present study will be supported by Tabriz University of Medical Sciences.
Author contribution
S-M and B-A drafted and designed the study. S-M would be carried out the study. AH-F, Y-Kh, M-A and M-AJ helped draft and revised the manuscript. S-M and B-A initiated the project. Y-Kh and M-AJ read the manuscript and provided feedback. All authors read and approved the final manuscript.
- Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med. 2013; 34: 465-484. PubMed: https://pubmed.ncbi.nlm.nih.gov/23266187/
- Bouillaud F. UCP2, not a physiologically relevant uncoupler but a glucose sparing switch impacting ROS production and glucose sensing. Biochim Biophys Acta. 2009; 1787: 377-83. PubMed: https://pubmed.ncbi.nlm.nih.gov/19413946/
- Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, et al. A signalling role for 4‐hydroxy‐2‐nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003; 22: 4103-4110. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC175801/
- Mehta SL, Li A. Neuroprotective role of mitochondrial uncoupling protein 2 in cerebral stroke. J Cereb Blood Flow Metab. 2009; 29: 1069-1078. PubMed: https://pubmed.ncbi.nlm.nih.gov/19240738/
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007; 132: 2169-2180. PubMed: https://pubmed.ncbi.nlm.nih.gov/17498510/
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009; 6: 399-409. PubMed: https://pubmed.ncbi.nlm.nih.gov/19399028/
- Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012; 56: 704-713. PubMed: https://pubmed.ncbi.nlm.nih.gov/22120206/
- Mathieu P, Lemieux I, Després JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010; 87: 407-416. PubMed: https://pubmed.ncbi.nlm.nih.gov/20200516/
- Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014; 15: 6184-6223. PubMed: https://pubmed.ncbi.nlm.nih.gov/24733068/
- Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015; 3: 207-215. PubMed: https://pubmed.ncbi.nlm.nih.gov/25066177/
- Ali AS, Ali S, Ahmad A, Bao B. Philip PA, et al. Expression of microRNAs: potential molecular link between obesity, diabetes and cancer. Obesity Reviews. 2011; 12: 1050-1062.
- Grossmann ME, Cleary MP. The balance between leptin and adiponectin in the control of carcinogenesis–focus on mammary tumorigenesis. Biochimie. 2012; 94: 2164-2171. PubMed: https://pubmed.ncbi.nlm.nih.gov/22728769/
- Divella R, De Luca R, Abbate I, Naglieri E, Daniele A, et al. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer. 2016; 7: 2346. PubMed: https://pubmed.ncbi.nlm.nih.gov/27994674/
- Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010; 15: 556-565. PubMed: https://pubmed.ncbi.nlm.nih.gov/20507889/
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004; 4: 579-591. PubMed: https://pubmed.ncbi.nlm.nih.gov/15286738/
- Van Kruijsdijk RC, Van Der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009; 18: 2569-2578. PubMed: https://pubmed.ncbi.nlm.nih.gov/19755644/
- Renehan AG, Roberts DL, Dive C, Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008; 114: 71-83. PubMed: https://pubmed.ncbi.nlm.nih.gov/18465361/
- Lee YH, Kim W, Yu BC, Park BL, Kim LH, et al. Association of the ins/del polymorphisms of uncoupling protein 2 (UCP2) with BMI in a Korean population. Biochem Biophys Res Commun. 2008; 371: 767-771. PubMed: https://pubmed.ncbi.nlm.nih.gov/18460338/
- Yoon Y, Park BL, Cha MH, Kim KS, Cheong HS, et al. Effects of genetic polymorphisms of UCP2 and UCP3 on very low calorie diet-induced body fat reduction in Korean female subjects. Biochem Biophys Res Commun. 2007; 359: 451-456. PubMed: https://pubmed.ncbi.nlm.nih.gov/17544366/
- Krempler F, Esterbauer H, Weitgasser R, Ebenbichler C, Patsch JR, et al. A functional polymorphism in the promoter of UCP2 enhances obesity risk but reduces type 2 diabetes risk in obese middle-aged humans. Diabetes. 2002; 51: 3331-3335. PubMed: https://pubmed.ncbi.nlm.nih.gov/12401727/
- Choi J, Kim KJ, Koh EJ, Lee BY. Gelidium elegans regulates the AMPK-PRDM16-UCP-1 pathway and has a synergistic effect with orlistat on obesity-associated features in mice fed a high-fat diet. Nutrients. 2017; 9: 342. PubMed: https://pubmed.ncbi.nlm.nih.gov/28358328/
- Mao L, et al. Long-chain polyunsaturated fatty acids and extensively hydrolyzed casein-induced browning in a Ucp-1 reporter mouse model of obesity. Food Funct. 2018; 9: 2362-2373. PubMed: https://pubmed.ncbi.nlm.nih.gov/29589625/
- Boss O, Hagen T, Lowell BB. Uncoupling proteins 2 and 3: potential regulators of mitochondrial energy metabolism. Diabetes. 2000; 49: 143-156. PubMed: https://pubmed.ncbi.nlm.nih.gov/10868929/
- Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, et al. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997; 15: 269-272. PubMed: https://pubmed.ncbi.nlm.nih.gov/9054939/
- Liu Y, Jiang H, He LY, Huang WJ, He XY, et al. Abnormal expression of uncoupling protein-2 correlates with CYP11A1 expression in polycystic ovary syndrome. Reprod Fertil Dev. 2011; 23: 520-526. PubMed: https://pubmed.ncbi.nlm.nih.gov/21557918/
- Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 2012; 10: e1001314. PubMed: https://pubmed.ncbi.nlm.nih.gov/22545021/
- Malli R, Graier WF. The Role of Mitochondria in the Activation/Maintenance of SOCE: The Contribution of Mitochondrial Ca(2+) Uptake, Mitochondrial Motility, and Location to Store-Operated Ca(2+) Entry. Adv Exp Med Biol. 2017; 993: 297-319. PubMed: https://pubmed.ncbi.nlm.nih.gov/28900921/
- Cannon B, Shabalina IG, Kramarova TV, Petrovic N, Nedergaard J. Uncoupling proteins: a role in protection against reactive oxygen species--or not? Biochim Biophys Acta. 2006; 1757: 449-458. PubMed: https://pubmed.ncbi.nlm.nih.gov/16806053/
- Nedergaard J, Cannon B. The 'novel' 'uncoupling' proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp Physiol. 2003; 88: 65-84. PubMed: https://pubmed.ncbi.nlm.nih.gov/12525856/
- Klaus S, Pültz S, Thöne-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes (Lond). 2005; 29: 615-623. PubMed: https://pubmed.ncbi.nlm.nih.gov/15738931/
- Nakayama K, Miyashita H, Yanagisawa Y, Iwamoto S. Seasonal effects of UCP1 gene polymorphism on visceral fat accumulation in Japanese adults. PLoS One. 2013; 8: e74720. PubMed: https://pubmed.ncbi.nlm.nih.gov/24086366/
- Porter RK. A new look at UCP 1. Biochim Biophys Acta. 2006; 1757: 446-448.
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, et al. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001; 1504: 82-106. PubMed: https://pubmed.ncbi.nlm.nih.gov/11239487/
- Esteves TC, Brand MD. The reactions catalysed by the mitochondrial uncoupling proteins UCP2 and UCP3. Biochim Biophys Acta. 2005; 1709: 35-44. PubMed: https://pubmed.ncbi.nlm.nih.gov/16005426/
- Rousset S, Mozo J, Dujardin G, Emre Y, Masscheleyn S, et al. UCP2 is a mitochondrial transporter with an unusual very short half‐life. FEBS Lett. 2007; 581: 479-482. PubMed: https://pubmed.ncbi.nlm.nih.gov/17240372/
- Zaninovich A. Role of uncoupling proteins UCP1, UCP2 and UCP3 in energy balance, type 2 diabetes and obesity. Synergism with the thyroid. Medicina. 2005; 65: 163-169. PubMed: https://pubmed.ncbi.nlm.nih.gov/16075814/
- Hassan NE, El-Masry SA, Zarouk W, El Banna EA, Mosaad RM, et al. Obesity phenotype in relation to gene polymorphism among samples of Egyptian children and their mothers. Genes Dis. 2018; 5: 150-157. PubMed: https://pubmed.ncbi.nlm.nih.gov/30258944/
- Vogler S, Goedde R, Miterski B, Gold R, Kroner A, et al. Association of a common polymorphism in the promoter of UCP2 with susceptibility to multiple sclerosis. J Molecular Med. 2005; 83: 806-811. PubMed: https://pubmed.ncbi.nlm.nih.gov/16021520/
- Rudofsky G, Schroedter A, Schlotterer A, Voron'ko OE, Schlimme M, et al. Functional polymorphisms of UCP2 and UCP3 are associated with a reduced prevalence of diabetic neuropathy in patients with type 1 diabetes. Diabetes Care. 2006; 29: 89-94. PubMed: https://pubmed.ncbi.nlm.nih.gov/16373902/
- Hurtaud C, Gelly C, Bouillaud F, Lévi-Meyrueis C. Translation control of UCP2 synthesis by the upstream open reading frame. Cell Mol Life Sci. 2006; 63: 1780-1789. PubMed: https://pubmed.ncbi.nlm.nih.gov/16845607/
- Beck V, Jabůrek M, Demina T, Rupprecht A, Porter RK, et al. Polyunsaturated fatty acids activate human uncoupling proteins 1 and 2 in planar lipid bilayers. FASEB J. 2007; 21: 1137-1144. PubMed: https://pubmed.ncbi.nlm.nih.gov/17242157/
- Reilly JM, Thompson MP. Dietary fatty acids up-regulate the expression of UCP2 in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 2000; 277: 541-545. PubMed: https://pubmed.ncbi.nlm.nih.gov/11061990/
- Rieusset J, Auwerx J, Vidal H. Regulation of gene expression by activation of the peroxisome proliferator-activated receptor γ with rosiglitazone (BRL 49653) in human adipocytes. Biochem Biophys Res Commun. 1999; 265: 265-271. PubMed: https://pubmed.ncbi.nlm.nih.gov/10548525/
- Bugge A, Siersbaek M, Madsen MS, Göndör A, Rougier C, et al. A novel intronic peroxisome proliferator-activated receptor γ enhancer in the Uncoupling Protein (UCP) 3 gene as a regulator of both UCP2 and-3 expression in adipocytes. J Bio Chem. 2010. 285: 17310-17317. PubMed: https://pubmed.ncbi.nlm.nih.gov/20360005/
- Oberkofler H, Klein K, Felder TK, Krempler F, Patsch W, et al. Role of peroxisome proliferator-activated receptor-γ coactivator-1α in the transcriptional regulation of the human uncoupling protein 2 gene in INS-1E cells. Endocrinology. 2006; 147: 966-976. PubMed: https://pubmed.ncbi.nlm.nih.gov/16282353/
- Lin J, Yang R, Tarr PT, Wu P, Handschin C, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell. 2005; 120: 261-273. PubMed: https://pubmed.ncbi.nlm.nih.gov/15680331/
- Medvedev AV, Robidoux J, Bai X, Cao W, Floering LM, et al. Regulation of the uncoupling protein-2 gene in INS-1 β-cells by oleic acid. J Biol Chem. 2002; 277: 42639-42644. PubMed: https://pubmed.ncbi.nlm.nih.gov/12205102/
- Azzu V, Brand MD. The on-off switches of the mitochondrial uncoupling proteins. Trends Biochem Sci. 2010. 35: 298-307. PubMed: https://pubmed.ncbi.nlm.nih.gov/20006514/
- Mailloux RJ, Seifert EL, Bouillaud F, Aguer C, Collins S, et al. Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem. 2011; 286: 21865-21875. PubMed: https://pubmed.ncbi.nlm.nih.gov/21515686/
- Chen H, Chan DC. Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab. 2017; 26: 39-48. PubMed: https://pubmed.ncbi.nlm.nih.gov/28648983/
- Richard D, Rivest R, Huang Q, Bouillaud F, Sanchis D, et al. Distribution of the uncoupling protein 2 mRNA in the mouse brain. J Comp Neurol. 1998; 397: 549-560. PubMed: https://pubmed.ncbi.nlm.nih.gov/9699915/
- Barnstable CJ, Reddy R, Li H, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) regulates retinal ganglion cell number and survival. J Molecular Neurosci. 2016; 58: 461-469. PubMed: https://pubmed.ncbi.nlm.nih.gov/26846222/
- Chen X, Wang K, Chen J, Guo J, Yin Y, et al. In vitro evidence suggests that miR-133a-mediated regulation of uncoupling protein 2 (UCP2) is an indispensable step in myogenic differentiation. J Biol Chem. 2009; 284: 5362-5369. PubMed: https://pubmed.ncbi.nlm.nih.gov/19073597/
- Sun LL, Jiang BG, Li WT, Zou JJ, Shi YQ, et al. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res Clin Pract. 2011; 91: 94-100. PubMed: https://pubmed.ncbi.nlm.nih.gov/21146880/
- Di Castro S, Scarpino S, Marchitti S, Bianchi F, Stanzione R, et al. Differential modulation of uncoupling protein 2 in kidneys of stroke-prone spontaneously hypertensive rats under high-salt/low-potassium diet. Hypertension. 2013; 61: 534-541. PubMed: https://pubmed.ncbi.nlm.nih.gov/23297375/
- Jin X, Chen D, Zheng RH, Zhang H, Chen YP, et al. miRNA-133a-UCP2 pathway regulates inflammatory bowel disease progress by influencing inflammation, oxidative stress and energy metabolism. World J Gastroenterol. 2017; 23: 76-86. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5221288/
- Jiang L, Qiu W, Zhou Y, Wen P, Fang L, et al. A microRNA-30e/mitochondrial uncoupling protein 2 axis mediates TGF-β1-induced tubular epithelial cell extracellular matrix production and kidney fibrosis. Kidney Int. 2013; 84: 285-296. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3731557/
- Muhammad HFL, Sulistyoningrum DC, Huriyati E, Lee YY, Wan Muda WAM. The Interaction between Coffee: Caffeine Consumption, UCP2 Gene Variation, and Adiposity in Adults-A Cross-Sectional Study. J Nutriti Metabol. 2019; 2019: 9606054. PubMed: https://pubmed.ncbi.nlm.nih.gov/30719347/
- Emre Y, Hurtaud C, Nübel T, Criscuolo F, Ricquier D, et al. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J. 2007; 402: 271-278. PubMed: https://pubmed.ncbi.nlm.nih.gov/17073824/
- Tagen M, Elorza A, Kempuraj D, Boucher W, Kepley CL, et al. Mitochondrial uncoupling protein 2 inhibits mast cell activation and reduces histamine content. J Immunol. 2009; 183: 6313-6319. PubMed: https://pubmed.ncbi.nlm.nih.gov/19846869/
- Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, et al. Persistent nuclear factor-κB activation in Ucp2-/-mice leads to enhanced nitric oxide and inflammatory cytokine production. J Bio Chem. 2005; 280: 19062-19069. PubMed: https://pubmed.ncbi.nlm.nih.gov/15757894/
- Su J, Liu J, Yan XY, Zhang Y, Zhang JJ, et al. Cytoprotective effect of the UCP2-SIRT3 signaling pathway by decreasing mitochondrial oxidative stress on cerebral ischemia–reperfusion injury. Int J Molecular Sci. 2017; 18: 1599. PubMed: https://pubmed.ncbi.nlm.nih.gov/28737710/
- Boschmann M, Thielecke F. The effects of epigallocatechin-3-gallate on thermogenesis and fat oxidation in obese men: a pilot study. J Am College Nutrit. 2007; 26: 389S-395S. PubMed: https://pubmed.ncbi.nlm.nih.gov/17906192/
- Schutz Y, Bessard T, Jequier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr. 1984; 40: 542-552. PubMed: https://pubmed.ncbi.nlm.nih.gov/6540980/
- Diepvens K, Westerterp KR, Westerterp-Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am J Physiol Regulat Integrat Comparat Phy. 2007; 292: R77-R85. PubMed: https://pubmed.ncbi.nlm.nih.gov/16840650/
- Tentolouris N, et al. Diet-induced thermogenesis and substrate oxidation are not different between lean and obese women after two different isocaloric meals, one rich in protein and one rich in fat. Metabolism. 2008; 57: 313-320. PubMed: https://pubmed.ncbi.nlm.nih.gov/18249201/
- Toda C, Diano S, Mitochondrial UCP2 in the central regulation of metabolism. Best Pract Res Clin Endocrinol Metab. 2014. 28: 757-764. PubMed: https://pubmed.ncbi.nlm.nih.gov/25256770/
- Dmitriev RI, Papkovsky DB. In vitro ischemia decreases histone H4K16 acetylation in neural cells. FEBS Letters. 2015; 589: 138-144. PubMed: https://pubmed.ncbi.nlm.nih.gov/25479088/
- Mattiasson G, et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003; 9: 1062-1068. PubMed: https://pubmed.ncbi.nlm.nih.gov/12858170/
- Myers Jr, MG, Olson DP. Central nervous system control of metabolism. Nature. 2012; 491: 357. PubMed: https://pubmed.ncbi.nlm.nih.gov/23151578/
- Horvath TL, Warden CH, Hajos M, Lombardi A, Goglia F, et al. Brain uncoupling protein 2: uncoupled neuronal mitochondria predict thermal synapses in homeostatic centers. J Neurosci. 1999; 19: 10417-10427. PubMed: https://pubmed.ncbi.nlm.nih.gov/10575039/
- Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, et al. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metabol. 2007; 5: 21-33. PubMed: https://pubmed.ncbi.nlm.nih.gov/17189204/
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, β cell dysfunction, and type 2 diabetes. Cell. 2001; 105: 745-755. PubMed: https://pubmed.ncbi.nlm.nih.gov/11440717/
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007; 449: 228-232. PubMed: https://pubmed.ncbi.nlm.nih.gov/17728716/
- Allister EM, Robson-Doucette CA, Prentice KJ, Hardy AB, Sultan S, et al. UCP2 regulates the glucagon response to fasting and starvation. Diabetes. 2013; 62: 1623-1633. PubMed: https://pubmed.ncbi.nlm.nih.gov/23434936/
- Diano S, Liu ZW, Jeong JK, Dietrich MO, Ruan HB, et al. Peroxisome proliferation–associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med. 2011; 17: 1121-1127. PubMed: https://pubmed.ncbi.nlm.nih.gov/21873987/
- Dulloo AG, Seydoux J, Jacquet J. Adaptive thermogenesis and uncoupling proteins: a reappraisal of their roles in fat metabolism and energy balance. Physiol Behav. 2004; 83: 587-602. PubMed: https://pubmed.ncbi.nlm.nih.gov/15621064/
- Rafiq R, El Haddaoui H, de Mutsert R, Rosendaal FR, Hiemstra PS, et al. Adiposity is a confounding factor which largely explains the association of serum vitamin D concentrations with C-reactive protein, leptin and adiponectin. Cytokine. 2020; 131: 155104. PubMed: https://pubmed.ncbi.nlm.nih.gov/32325367/
- Rezazadeh L, Gargari BP, Jafarabadi MA, Alipour B. Effects of probiotic yogurt on glycemic indexes and endothelial dysfunction markers in patients with metabolic syndrome. Nutrition. 2019; 62: 162-168. PubMed: https://pubmed.ncbi.nlm.nih.gov/30921552/
- Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González A, et al. Inflammation, oxidative stress, and obesity. Int J Molecular Sci. 2011; 12: 3117-3132. PubMed: https://pubmed.ncbi.nlm.nih.gov/21686173/
- Chan JL, Heist K, De Paoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Investigat. 2003; 111: 1409-1421. PubMed: https://pubmed.ncbi.nlm.nih.gov/12727933/
- Wasim M, Awan FR, Najam SS, Khan AR, Khan HN. Role of leptin deficiency, inefficiency, and leptin receptors in obesity. Biochem Genetics. 2016; 54: 565-572. PubMed: https://pubmed.ncbi.nlm.nih.gov/27313173/
- Scarpace P, Nicolson M, Matheny M. UCP2, UCP3 and leptin gene expression: modulation by food restriction and leptin. J Endocrinol. 1998; 159: 349-357. PubMed: https://pubmed.ncbi.nlm.nih.gov/9795377/
- Ho WL, Liu HF, Ho HW, Zhang WY, Chu AC, et al. Mitochondrial uncoupling protein-2 (UCP2) mediates leptin protection against MPP+ toxicity in neuronal cells. Neurotox Res. 2010; 17: 332-343. PubMed: https://pubmed.ncbi.nlm.nih.gov/19763737/
- Guo JJ, Liu YJ, Li MX, Yang YJ, Recker RR, et al. Linkage exclusion analysis of two candidate regions on chromosomes 7 and 11: leptin and UCP2/UCP3 are not QTLs for obesity in US Caucasians. Biochem Biophys Res Commn. 2005; 332: 602-608.
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commn. 1999; 257: 79-83. PubMed: https://pubmed.ncbi.nlm.nih.gov/10092513/
- Hotta, K, Funahashi T, Arita Y, Takahashi M, Matsuda M, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000; 20: 1595-1599. PubMed: https://pubmed.ncbi.nlm.nih.gov/10845877/
- Brand C, Gaya ACA, Dias AF, Agostinis-Sobrinho C, Farinha JB, et al. Relationship between insulin resistance and adipocytokines: the mediator role of adiposity in children. Annals of Human Biology. 2020; 47: 244-249. PubMed: https://pubmed.ncbi.nlm.nih.gov/32279531/
- Zhou M, Xu A, Tam PKH, Lam KSL, Huang B, et al. Upregulation of UCP2 by adiponectin: the involvement of mitochondrial superoxide and hnRNP K. PLoS One. 2012; 7: e32349. PubMed: https://pubmed.ncbi.nlm.nih.gov/22359684/
- Taghadomi Masoumi Z, Eshraghian MR, Hedayati M, Pishva H. Association between uncoupling protein 2, adiponectin and resting energy expenditure in obese women with normal and low resting energy expenditure. Gynecol Endocrinol. 2018; 34: 166-170. PubMed: https://pubmed.ncbi.nlm.nih.gov/29017362/
- Mahadik SR, Lele RD, Saranath D, Seth A, Parikh V. Uncoupling protein-2 (UCP2) gene expression in subcutaneous and omental adipose tissue of Asian Indians: relationship to adiponectin and parameters of metabolic syndrome. Adipocyte. 2012; 1: 101-107. PubMed: https://pubmed.ncbi.nlm.nih.gov/23700519/
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002; 415: 96-99. PubMed: https://pubmed.ncbi.nlm.nih.gov/11780125/
- Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci USA. 2014; 111: 960-965. PubMed: https://pubmed.ncbi.nlm.nih.gov/24395786/
- Pishva H. UCP2, SHBG, Leptin, and T3 Levels Are Associated with Resting Energy Expenditure in Obese Women. Endocr Metab Immune Disord Drug Targets. 2019.
- Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, et al. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J. 2008; 22: 9-18. PubMed: https://pubmed.ncbi.nlm.nih.gov/17855623/
- B Andrews, Z, Uncoupling protein-2 and the potential link between metabolism and longevity. Curr Aging Sci. 2010; 3: 102-112. PubMed: https://pubmed.ncbi.nlm.nih.gov/20158496/
- Cheng WC, Tsui YC, Ragusa S, Koelzer VH, Mina M, et al. Uncoupling protein 2 reprograms the tumor microenvironment to support the anti-tumor immune cycle. Nat Immunol. 2019; 20: 206-217. PubMed: https://pubmed.ncbi.nlm.nih.gov/30664764/
- Rask-Andersen M, Olszewski PK, Levine AS, Schiöth HB. Molecular mechanisms underlying anorexia nervosa: focus on human gene association studies and systems controlling food intake. Brain Res Rev. 2010; 62: 147-164. PubMed: https://pubmed.ncbi.nlm.nih.gov/19931559/
- Esteves P, Pecqueur C, Ransy C, Esnous C, Lenoir V, et al. Mitochondrial retrograde signaling mediated by UCP2 inhibits cancer cell proliferation and tumorigenesis. Cancer Res. 2014; 74: 3971-3982. PubMed: https://pubmed.ncbi.nlm.nih.gov/24853548/ B
- Schonfeld-Warden NA, Warden CH. Physiological effects of variants in human uncoupling proteins: UCP2 influences body-mass index. Biochem Soc Trans. 2001: 29: 777-784. PubMed: https://pubmed.ncbi.nlm.nih.gov/11709074/
- Mutombo B, Yamasaki M, Shiwaku K, UCP2 I/D modulated change in BMI during a lifestyle modification intervention study in Japanese subjects. Genet Test Mol Biomarkers. 2013; 17: 16-20. PubMed: https://pubmed.ncbi.nlm.nih.gov/23101559/
- Liu X, Zhang B, Liu X, Shen Y, Li J,, et al. A 45-bp insertion/deletion polymorphism in uncoupling protein 2 is not associated with obesity in a Chinese population. Biochem Genet. 2012; 50: 784-796. PubMed: https://pubmed.ncbi.nlm.nih.gov/22733179/
- Cortes-Oliveira C, Nicoletti CF, de Souza Pinhel MA, de Oliveira BAP, Quinhoneiro DCG, et al. UCP2 expression is associated with weight loss after hypocaloric diet intervention. Eur J Clin Nutr. 2017; 71: 402-406. PubMed: https://pubmed.ncbi.nlm.nih.gov/27759071/
- Kim YJ. A mixture of the aqueous extract of Garcinia cambogia, soy peptide and L: -carnitine reduces the accumulation of visceral fat mass in rats rendered obese by a high fat diet. Genes Nutr. 2008; 2: 353-358.
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010; 49: 1603-1616. PubMed: https://pubmed.ncbi.nlm.nih.gov/20840865/
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation. 2017; 114: 1752-1761. PubMed: https://pubmed.ncbi.nlm.nih.gov/15599400/
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-biological interactions. 2006; 160: 1-40. PubMed: https://pubmed.ncbi.nlm.nih.gov/16430879/
- Kizaki T, Suzuki K, Hitomi Y, Taniguchi N, Saitoh D, et al. Uncoupling protein 2 plays an important role in nitric oxide production of lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci. 2002; 99: 9392-9397. PubMed: https://pubmed.ncbi.nlm.nih.gov/12089332/
- Ryu JW, Hong KH, Maeng JH, Kim JB, Ko J, et al. Overexpression of uncoupling protein 2 in THP1 monocytes inhibits β2 integrin-mediated firm adhesion and transendothelial migration. Arteriosclerosis, thrombosis, and vascular biology. 2004; 24: 864-870. PubMed: https://pubmed.ncbi.nlm.nih.gov/15016641/
- Nègre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, et al. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997; 11: 809-815. PubMed: https://pubmed.ncbi.nlm.nih.gov/9271366/
- Marseglia L, Manti S, D'Angelo G, Nicotera A, Parisi E, et al. Oxidative stress in obesity: a critical component in human diseases. International journal of molecular sciences. 2015; 16: 378-400. PubMed: https://pubmed.ncbi.nlm.nih.gov/25548896/
- Huizing M, Ruitenbeek W, van den Heuvel LP, Dolce V, Iacobazzi V, et al. Human mitochondrial transmembrane metabolite carriers: tissue distribution and its implication for mitochondrial disorders. J Bioenerg Biomembr. 1998; 30: 277-284. PubMed: https://pubmed.ncbi.nlm.nih.gov/9733094/
- Cardel MI, Jastreboff AM, Kelly AS, Treatment of adolescent obesity in 2020. JAMA. 2019; 322: 1707-1708. PubMed: https://pubmed.ncbi.nlm.nih.gov/31566652/
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014; 384: 766-781. PubMed: https://pubmed.ncbi.nlm.nih.gov/24880830/
- Gadde KM, Martin CK, Berthoud HR, Heymsfield SB. Obesity. Pathophysiology and Management. 2018; 71: 69-84. PubMed: https://pubmed.ncbi.nlm.nih.gov/29301630/
- Tereshin, E, A role of fatty acids in the development of oxidative stress in aging. A hypothesis. Adv Gerontol. 2007; 20: 59-65. PubMed: https://pubmed.ncbi.nlm.nih.gov/17969588/
- Stępień M, Stępień A, Wlazeł RN, Paradowski M, Banach M, et al. Obesity indices and inflammatory markers in obese non-diabetic normo-and hypertensive patients: a comparative pilot study. Lipids Health Dis. 2014; 13: 29. PubMed: https://pubmed.ncbi.nlm.nih.gov/24507240/
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011; 29: 415-445. PubMed: https://pubmed.ncbi.nlm.nih.gov/21219177/
- Pihl E, Zilmer K, Kullisaar T, Kairane C, Mägi A, et al. Atherogenic inflammatory and oxidative stress markers in relation to overweight values in male former athletes. Int Jo Obes. 2006; 30: 141-146. PubMed: https://pubmed.ncbi.nlm.nih.gov/16158088/
- Vial G, Dubouchaud H, Couturier K, Cottet-Rousselle C, Taleux N, et al. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J Hepatol. 2011; 54: 348-356. PubMed: https://pubmed.ncbi.nlm.nih.gov/21109325/
- da Costa RM, Fais RS, Dechandt CRP, Louzada-Junior P, Alberici LC, et al. Increased mitochondrial ROS generation mediates the loss of the anti‐contractile effects of perivascular adipose tissue in high‐fat diet obese mice. Br J Pharmacol. 2017; 174: 3527-3541. PubMed: https://pubmed.ncbi.nlm.nih.gov/27930804/
- Xu H, Li F. miR127 aggravates myocardial failure by promoting the TGFbeta1/Smad3 signaling. Mol Med Rep. 2018; 18: 4839-4846. PubMed: https://pubmed.ncbi.nlm.nih.gov/30272299/
- Włodarczyk M, Nowicka G. Obesity, DNA damage, and development of obesity-related diseases. Int J Mole Sci. 2019; 20: 1146. PubMed: https://pubmed.ncbi.nlm.nih.gov/30845725/
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005; 115: 1111-1119. PubMed: https://pubmed.ncbi.nlm.nih.gov/15864338/
- Hanley AJ, Festa A, D’Agostino, Jr. RB, Wagenknecht LE, et al. Metabolic and inflammation variable clusters and prediction of type 2 diabetes: factor analysis using directly measured insulin sensitivity. Diabetes. 2004; 53: 1773-1781.
- Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. 2015; 13: 423-444. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4808277/
- Kim JD, Yoon NA, Jin S, Diano S. Microglial UCP2 mediates inflammation and obesity induced by high-fat feeding. Cell metabolism. 2019; 30: 952-962. PubMed: https://pubmed.ncbi.nlm.nih.gov/31495690/
- Jiang Z, Yang Q, Zhu C. UCP2 in early diagnosis and prognosis of sepsis. Eur Rev Med Pharmacol Sci. 2017; 21: 549-553. PubMed: https://pubmed.ncbi.nlm.nih.gov/28239813/
- McMurray F, Patten DA, Harper ME. Reactive oxygen species and oxidative stress in obesity—recent findings and empirical approaches. Obesity. 2016; 24: 2301-2310. PubMed: https://pubmed.ncbi.nlm.nih.gov/27804267/
- Mori S, Yoshizuka N, Takizawa M, Takema Y, Murase T, et al. Expression of uncoupling proteins in human skin and skin-derived cells. J Invest Dermatol. 2008; 128: 1894-1900. PubMed: https://pubmed.ncbi.nlm.nih.gov/18305572/
- Li W, Zhang C, Jackson K, Shen X, Jin R, et al. UCP2 knockout suppresses mouse skin carcinogenesis. Cancer Prevention Research. 2015; 8: 487-491. PubMed: https://pubmed.ncbi.nlm.nih.gov/25784177/
- Emre Y, Nubel T. Uncoupling protein UCP2: when mitochondrial activity meets immunity. FEBS Lett. 2010; 584: 1437-1442. PubMed: https://pubmed.ncbi.nlm.nih.gov/20227410/